В настоящее время ожирение следует рассматривать не просто как косметическую проблему, а как эпидемию, угрожающую благополучию людей во всем мире. По данным ВОЗ, в 2016 г. более 1,9 млрд взрослых от 18 лет и старше имели избыточный вес, из них более 650 млн страдали ожирением [1]. Патологии, связанные с ожирением, такие как сахарный диабет (СД) 2 типа, сердечно-сосудистые заболевания, остеоартрит и некоторые формы рака, представляют собой серьезную угрозу для здоровья населения.
Неалкогольная жировая болезнь печени (НАЖБП) в настоящее время рассматривается как одно из связанных с метаболическим синдромом заболеваний или одно из проявлений метаболического синдрома, ассоциированное с ожирением и инсулинорезистентностью [2, 3]. Ожирение, и особенно висцеральная его форма, является наиболее распространенным и хорошо доказанным фактором риска развития НАЖБП. Так, большинство (>95%) пациентов с тяжелым ожирением, подвергшихся бариатрическим операциям, имело клинические проявления НАЖБП [4, 5].
Лептин – гормон жировой ткани, секретируемый адипоцитами и представляющий собой сигнал для отрицательной обратной связи в центральной нервной системе (ЦНС), регулирующий аппетит, обмен веществ и половое созревание [6]. Лептин секретируется в основном белой жировой тканью и служит сигналом для мозга об энергетических запасах организма, а также секретируется в небольшом количестве другими тканями, включая костный мозг, яичники, плаценту, желудок и лимфоидную ткань [7]. За счет снижения потребления пищи и увеличения термогенеза лептин контролирует количество жировой ткани и, следовательно, массу тела. Секреция лептина регулируется различными гормонами, в том числе инсулином, глюкокортикоидами и самим лептином [7, 8]. Лептин секретируется пропорционально количеству белой жировой ткани и присутствует в обращении либо в свободной форме, либо в форме, связанной с белками [9]. Он играет решающую роль в регуляции энергетического гомеостаза, метаболизма глюкозы и липидов, репродукции и нейроэндокринных функций [8, 10].
Действие лептина в периферических тканях осуществляется путем взаимодействия со специфическими трансмембранными рецепторами. Рецептор лептина (Lep-R или Ob-R) принадлежит к семейству цитокинов 1 класса и может быть важным определителем чувствительности к лептину [11]. Выделено шесть изоформ рецепторов к этому гормону, которые экспрессируются в ЦНС и периферических тканях [12]. Растворимая форма рецептора лептина (sLep-R) служит основным лептин-связывающим белком, формирующим комплексы с циркулирующим лептином. Предполагают, что именно баланс между свободной и связанной формами лептина регулирует его биодоступность [13]. Также существует мнение, что присутствующая при ожирении резистентность к лептину связана с нарушением переноса гормона транспортными белками или растворимыми рецепторами лептина [14]. Однако точная патофизиологическая роль sLep-R еще не определена.
Клинические данные о циркулирующих уровнях лептина у пациентов с НАЖБП противоречивы [15]. Выяснение роли лептина и его растворимого рецептора у больных НАЖБП, ассоциированной с ожирением, может иметь важное клиническое значение, так как имеется значительная потребность в биомаркерах жировой болезни печени и особенно в маркерах неалкогольного стеатогепатита, а также в разработке методов таргетной терапии [15].
Целью исследования стало определение клинического значения содержания лептина в крови больных НАЖБП, ассоциированной с ожирением.
МАТЕРИАЛ И МЕТОДЫ
Обследовано 114 пациентов с НАЖБП (56 женщин и 58 мужчин, средний возраст 51,4±1,34 лет). У всех больных имелось ожирение или избыточная масса тела, средний показатель индекса массы тела (ИМТ) составил 35,46±0,92 кг/м2. Показатели обмена липидов отличались от нормы: общий холестерин – 5,83±0,23 ммоль/л, ХС ЛПНП – 3,6±0,22 ммоль/л, ХС ЛПВП – 1,08±0,04 ммоль/л, триглицериды – 2,85±0,35 ммоль/л. Уровень активности цитолитических ферментов при НАЖБП составил: АлАТ – 61,0 (39,5–93,8) Ед/л, АсАТ – 40,0 (29,5–58,5) Ед/л. У всех пациентов с НАЖБП имело место наличие инсулинорезистентности – показатели НОМА-индекса в группе равнялись 4,75 (4,14–7,32).
35 пациентов (31%) имели сопутствующую артериальную гипертензию (АГ), у 28 (24%) диагностировали СД 2 типа. У 42 больных был установлен диагноз неалкогольного стеатогепатита (НАСГ), в остальных случаях определялся стеатоз печени.
В исследование не включались пациенты с сопутствующим поражением печени алкогольной этиологии (употребление алкоголя в гепатотоксических дозах), с наличием маркеров вирусных гепатитов – anti-HCV, HBsAg, с аутоиммунным или лекарственным поражением печени, а также при наличии СД 1 типа или инсулинопотребного СД 2 типа. Также к критериям исключения относился суб- и декомпенсированный цирроз печени (классы B и C по Чайлд–Пью).
Группа сравнения состояла из 42 здоровых лиц с ИМТ <25 и была сопоставима с больными по полу и возрасту.
Всем пациентам выполнялось общеклиническое исследование с обязательным определением антропометрических параметров (ИМТ, объем талии (ОТ), объем бедер (ОБ), соотношение ОТ/ОБ), а также стандартное лабораторно-инструментальное обследование. 37 пациентам была выполнена пункционная биопсия печени. Степень стеатоза по материалам гистологического исследования и стадию процесса (выраженность фиброза) оценивали согласно Е.М. Brunt.
В плазме крови больных определяли содержание Lep-R и Lep методом иммуноферментного анализа (ИФА) с помощью коммерческих тест-систем (ВioVendor и Diagnostics Biochem Canada Inc.). Вычисляли индекс свободного лептина как соотношение Lep/Lep-R.
Все результаты были статистически обработаны с помощью компьютерных программ Microsoft Office Excel 2010 со встроенной подпрограммой Attestat 10.5.1, IBM SPSS Statistics 21. При нормальном распределении данных количественные значения представлены в виде средней ± стандартной ошибки средней (Х±Sx). Вычисление критерия Стьюдента использовалось при сравнительном анализе у групп с нормальным распределением. Данные выборок с ненормальным распределением описывались в виде медианы и интерквантильного (25 и 75 процентили) размаха – Ме (Q1–Q3). Для сравнения данных в этих группах использовали U-критерий Манна–Уитни. Достоверными считали различия при p ≤0,05. Корреляционный анализ проводился с определением критерия Спирмена.
РЕЗУЛЬТАТЫ И ОБСУЖДЕНИЕ
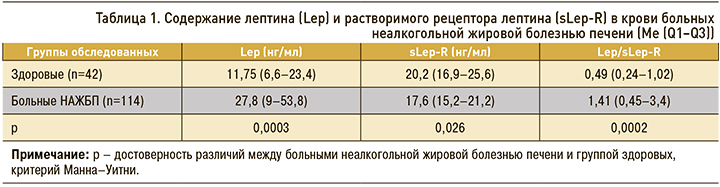
Содержание лептина в крови пациентов с НАЖБП было выше, чем в группе сравнения (табл. 1). При этом плазменная концентрация в крови sLep-R у больных НАЖБП, напротив, была ниже, чем у здоровых лиц. Соотношение значений лептина к уровню его растворимого рецептора – индекс свободного лептина (Lep/sLep-R) – почти в 3 раза превышал аналогичный показатель у здоровых лиц. Метаанализ 33 исследований, опубликованных в период с 1999 по 2014 г. (всего 2612 человек), проведенный A.T. Polyzos и соавт., показал, что, несмотря на некоторую разнородность полученных результатов, у пациентов с НАЖБП отмечаются более высокие уровни циркулирующего лептина относительно групп контроля [15]. Известно, что sLep-R обеспечивает связь свободных фракций лептина в плазме и регулирует его биодоступность [16]. Повышение индекса свободного лептина у пациентов может свидетельствовать об имеющейся у них лептинорезистентности [14, 17]. Повышенная продукция лептина, сопровождающаяся снижением концентрации sLep-R, указывает на высокую резистентность периферических тканей к действию лептина [17]. Имеются данные, что лептинорезистентность проявляется в большей степени у пациентов с НАЖБП, чем у лиц с ожирением без стеатоза печени [17], а также известно, что уровни сывороточного лептина повышены при НАСГ [18, 19]. В то же время концентрацию sLep-R находили значительно пониженной у пациентов с НАЖБП по сравнению с контрольной группой [17].
Нами была также выявлена обратная корреляционная зависимость между уровнями Lep и sLep-R у пациентов в группе с НАЖБП: rs= –0,61, р=0,0005. Эта зависимость свидетельствует об имеющемся дисбалансе системы «лептин – рецептор лептина». Существует мнение, что к сниженной продукции рецептора лептина может приводить полиморфизм его гена. Так, было показано, что полиморфизмы в гене рецептора лептина связаны с риском развития неалкогольной жировой болезни печени [20, 21]. Считают, полиморфизм гена рецептора лептина способствует возникновению НАЖБП, влияя на липидный обмен и чувствительность к инсулину [22].
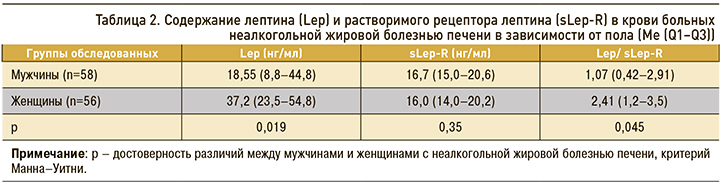
Концентрация лептина и его рецептора в крови не зависела от возраста больных, однако имелись определенные гендерные различия (табл. 2). Содержание лептина у женщин с НАЖБП было выше, чем у пациентов мужского пола, но уровни sLep-R не различались. Вместе с тем индекс свободного лептина у женщин все же превышал аналогичный показатель у мужчин, что свидетельствовало о большей выраженности лептинорезистентности. Это подтверждают и другие авторы [14, 17]. Лептин секретируется и экспрессируется преимущественно в жировой ткани и гендерная разница объясняется отчасти более высоким процентом жировых отложений у женщин.
Уровень лептина в крови имел прямую зависимость от ИМТ (rs=0,31), соотношения ОТ/ОБ (rs=0,34), показателей ХС ЛПНП (rs=0,29) и триглицеридов (rs=0,35) – р <0,05 во всех случаях. Выявленные корреляции подтверждают взаимосвязь гиперлептинемии с ожирением, а также с гиперлипидемией, ассоциируемой с липотоксичностью при НАЖБП [17, 23]. Кроме того, мы обнаружили положительную корреляционную зависимость между содержанием в плазме лептина и значениями НОМА-индекса: rs =0,39 (р=0,028). Известно, что Lep способен влиять на чувствительность гепатоцитов к сигналам инсулина, так как лептин способствует накоплению в клетках печени свободных жирных кислот, приводя в итоге к инсулинорезистентности [23]. В то же время нам не удалось обнаружить прямых корреляционных зависимостей между уровнями лептина и показателями биохимической активности – ферментами цитолиза.
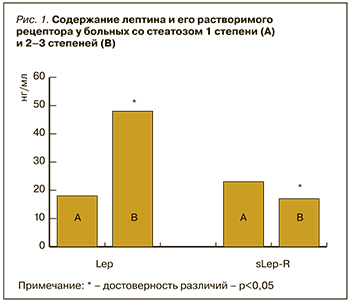
Было проанализировано содержание в плазме крови Lep и sLep-R в зависимости от результатов морфологического исследования биоптатов печени. Оказалось, что у 14 пациентов с 1-й степенью стеатоза (содержание жира <33%) уровень лептина был ниже, а содержание в крови растворимого рецептора выше, чем при стеатозе 2 и 3 степени (рис. 1).
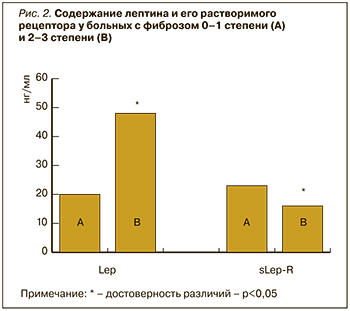 Ранее сообщалось, что повышенный уровень лептина может являться признаком стеатоза [17, 18, 22]. Кроме того, аналогичную зависимость Lep и sLep-R мы обнаружили и от степени фиброза. У 16 пациентов был диагностирован фиброз F-1 или отсутствие признаков фиброза, у 12 – F-2, а у 9 – F-3. Пациентам на стадии цирроза печени (F-4) в нашем исследовании биопсия печени не проводилась. На рис. 2 показано, что увеличение стадии фиброза (пациенты с фиброзом F-2 и F-3) сопровождалось нарастанием плазменного лептина и снижением концентрации sLep-R. Лептин способен усиливать фиброгенез в ответ на повреждение печени через трансформирующий фактор роста (TGF)-β1, влияя на клетки Купффера и печеночные звездчатые клетки, а также индуцируя выработку мощного провоспалительного цитокина ФНО-α [23, 24, 25]. Возможно, профиброгенному действию лептина способствует и дефицит протективного адипонектина у этих пациентов [23]. Сообщалось, что высокий уровень циркулирующего лептина был связан с тяжестью НАЖБП, так как избыток лептина может способствовать печеночному воспалению и развитию фиброза [9, 15].
Ранее сообщалось, что повышенный уровень лептина может являться признаком стеатоза [17, 18, 22]. Кроме того, аналогичную зависимость Lep и sLep-R мы обнаружили и от степени фиброза. У 16 пациентов был диагностирован фиброз F-1 или отсутствие признаков фиброза, у 12 – F-2, а у 9 – F-3. Пациентам на стадии цирроза печени (F-4) в нашем исследовании биопсия печени не проводилась. На рис. 2 показано, что увеличение стадии фиброза (пациенты с фиброзом F-2 и F-3) сопровождалось нарастанием плазменного лептина и снижением концентрации sLep-R. Лептин способен усиливать фиброгенез в ответ на повреждение печени через трансформирующий фактор роста (TGF)-β1, влияя на клетки Купффера и печеночные звездчатые клетки, а также индуцируя выработку мощного провоспалительного цитокина ФНО-α [23, 24, 25]. Возможно, профиброгенному действию лептина способствует и дефицит протективного адипонектина у этих пациентов [23]. Сообщалось, что высокий уровень циркулирующего лептина был связан с тяжестью НАЖБП, так как избыток лептина может способствовать печеночному воспалению и развитию фиброза [9, 15].
ЗАКЛЮЧЕНИЕ
У пациентов с НАЖБП, ассоциированной с ожирением, уровень лептина в крови и индекс свободного лептина выше, а содержание Lep-R ниже, чем у здоровых. Показатели Lep и Lep-R находятся в обратной зависимости. Содержание лептина и соотношение Lep/Lep-R у женщин с НАЖБП было выше, чем у пациентов мужского пола, но уровни sLep-R при этом не различались. Уровень лептина в крови имел прямую зависимость от ИМТ, соотношения ОТ/ОБ, значения НОМА-индекса, показателей ХС ЛПНП и триглицеридов. Выраженный стеатоз печени (2 и 3 степени), а также продвинутые стадии фиброза (F-2 и F-3) сопровождались нарастанием плазменного лептина и снижением концентрации sLep-R, что отражало важную роль лептина и лептинорезистентности в прогрессировании НАЖБП.



