ВВЕДЕНИЕ
Вирус гепатита дельта (HDV) вызывает относительно редкую, но агрессивную форму вирусного гепатита (гепатит D), которая развивается у пациентов, коинфицированных вирусом гепатита В (HBV). Поверхностные белки HBV (зачастую обозначаемые HBsAg) формируют инфекционную частицу HDV, поэтому наличие коинфекции этими вирусами – обязательное условие развития хронического вирусного гепатита D (ХГD).
ХГD требует эффективной терапии из-за неблагоприятного течения инфекции, быстрого прогрессирования в цирроз печени (ЦП), повышенной частоты печеночной декомпенсации и более высоких показателей смертности по сравнению с моноинфекцией HBV [1]. Клинико-вирусологические характеристики пациентов с HBV/HDV-инфекцией и подходы к ее лечению, несмотря на проводимые исследования, определены недостаточно.
Лечение ХГD представляет собой серьезную проблему, так как специфического ингибитора, способного подавлять репродукцию вируса, не существует. Противовирусные средства, влияющие на репликацию HDV, могут воздействовать на специфические факторы человека или вируса, причем последние действуют либо специфически на HDV, либо опосредованно за счет снижения продукции HBsAg. Препараты, способные действовать непосредственно на клеточные ферменты, катализирующие многие стадии цикла репликации HDV (такие как РНК-полимераза II или фарнезилтрансфераза), изучены мало [2].
Отдельной проблемой терапии ХГD является определение маркеров для прогнозирования клинического исхода заболевания и, следовательно, оценки ответа на лечение. Известно, что при моноинфекции HBV длительное подавление репликации ДНК HBV посредством противовирусных средств связано со снижением риска развития печеночных осложнений [3]. В случае же с больными ХГD отмечалось, что снижение уровня РНК HDV во время лечения препаратам интерферона альфа (ИФН-α) было связано с улучшением клинических результатов, даже если не удавалось достичь недетектируемого уровня РНК HDV [4, 5].
Из-за агрессивного прогрессирования ХГD до ЦП и гепатоцеллюлярной карциномы (ГЦК) первостепенной целью его терапии служит снижение риска развития неблагоприятных исходов, необходимости трансплантации печени и смерти пациента от причин, связанных с этим заболеванием. Конечные цели лечения – нормализация трансаминаз, исчезновение детектируемой РНК HDV и в конечном счете сероконверсия HBsAg [6]. Из-за отсутствия данных о длительности успешного подавления репликации вируса и трудностей в достижении клиренса HBsAg были предложены и приняты промежуточные суррогатные маркеры эффективности лечения ХГD: снижение уровня РНК HDV в сыворотке более чем на 2 lg МЕ/мл в сочетании с нормализацией уровня аланинаминотрансферазы (АЛТ). Данные маркеры были приняты в качестве меры оценки эффективности лечения в клинических испытаниях. В последние годы эти промежуточные и суррогатные терапевтические конечные точки позволили протестировать эффективность новых противовирусных средств при HDV-инфекции [7].
ПРЕПАРАТЫ ИНТЕРФЕРОНА
В течение 30 лет единственным препаратом, применяемым для лечения ХГD, оставался ИФН-α [8], а с 2006 г. было начато применение его пегилированой формы (ПЭГ-ИФН-α) [9]. Однако, как показывают клинические исследования, устойчивый вирусологический ответ (УВО) после применения препарата как в виде монотерапии, так и в сочетании с аналогами нуклеоз(т)идов (АН), подавляющими репликацию HBV, развивается не более чем в 23–47% случаев [10–13]. Результаты 10-летнего наблюдения продемонстрировали, что пациенты, получавшие ИФН-α2a (9 МЕ 3 раза/нед), имели значительно лучшую долгосрочную выживаемость и более выраженное подавление репликации HDV, чем те, кто получал более низкую дозу этого же лекарственного средства или не получал лечения. К сожалению, рецидив виремии HDV был обнаружен у всех пролеченных пациентов с помощью чувствительного ПЦР-теста. Важно добавить, что парные биопсии печени показали значительную и длительную регрессию фиброза печени спустя годы после терапии ИФН-α2a [14].
В исследовании эффективности ПЭГ-ИФН-α HIDIT-1 включение в схему лечения ингибитора репликации HBV адефовира привело к тому, что у 28% пациентов РНК HDV не определялась до 24 нед после лечения, но при более длительном наблюдении были отмечены поздние рецидивы с детектируемой РНК HDV у половины пациентов [15]. Низкий уровень эффективности интерферонотерапии нельзя считать неожиданным. Исследования на трансфицированных клеточных линиях, описанные Pugnale P. et al., показали общую нечувствительность репликации РНК HDV к ИФН-α [16], хотя эксперименты на гуманизированных мышах продемонстрировали различия в ответе на ИФН-α у разных штаммов HDV генотипа 1 [17]. В исследовании Zhang Z. et al. было показано, что интерфероны могут быть эффективны только на очень ранней стадии инфекции, когда вирус проникает в гепатоциты, но не на стадии установившейся внутриклеточной HDV-инфекции [18].
Для контроля прогрессирования заболевания, вызванного HBV, лекарственные средства из группы АН (например, тенофовир или энтекавир) рассматриваются как препараты выбора, поскольку успешно подавляют репликацию HBV [19]. Однако их воздействие непосредственно на HDV минимально. Поскольку АН не влияют напрямую на экспрессию поверхностных белков HBV, они не могут подавлять сборку вирионов HDV в инфицированных клетках. Более того, в связи с тем что эти препараты специфически действуют на обратную транскриптазу HBV, они не оказывают прямого действия на репликацию РНК HDV, осуществляемую клеточной РНК-полимеразой. Brancaccio G. et al. установили, что АН не влияют на виремию HDV, а длительное лечение этими препаратами не оказывает существенного влияния на клинический исход заболевания, вызванного HDV [20].
Итоги исследования HIDIT II свидетельствуют, что продление ПЭГ-ИФН-α в комбинации с тенофовиром до 24 мес не улучшило вирусологический ответ (ВО) и не предотвратило поздние рецидивы [21]. Обнаружено, что добавление энтекавира к ПЭГ-ИФН-α не способствует повышению частоты ВО за 24 нед лечения [22]. Тем не менее АН рекомендуются при наличии постоянной или периодически повышающейся вирусной нагрузки HBV >2000 МЕ/мл с целью предотвращения повышения уровней АЛТ, которые ускоряют прогрессирование повреждения печени [19].
В настоящее время более чем 25-летний опыт лечения ХГD препаратами ИФН можно резюмировать следующим образом: ИФН-α может ингибировать репликацию HDV и уменьшать воспаление печени и прогрессирование заболевания у 20–25% пациентов; монотерапия или добавление АН не оказывает необходимого влияния, но может предотвратить репликацию HBV; терапия ИФН противопоказана при наиболее запущенных формах заболеваниях печени, имеет значительные побочные эффекты и может вызывать ассоциированный аутоиммунный гепатит. По всем этим причинам существует настоятельная необходимость в более безопасных и эффективных методах лечения, подавляющих HDV.
НОВЫЕ НАПРАВЛЕНИЯ В ЛЕЧЕНИИ ГЕПАТИТА ДЕЛЬТА
Разрабатываемые в настоящее время методы лечения ХГD в основном нацелены на блокирование входа вируса в клетку, подавление секреции HBsAg, предотвращение образования репликативных промежуточных продуктов кзкДНК HBV или РНК HDV для прямого или косвенного воздействия на жизненный цикл HDV [9, 23, 24].
Интерферон лямбда
С учетом побочных эффектов, вызываемым ИФН-α, в лечении ХГD появилась альтернатива – использовать ИФН-λ для лечения пациентов с HDV, не отвечающих критериям назначения ИФН-α. Обоснованием для применения ИФН-λ стали:
- локализация цели – рецептор ИФН-λ (IFNLR1) экспрессируется в эпителиальных тканях легких, печени и кишечника, в отличие от ИФН-α, чей рецептор (IFNAR1/2) повсеместно экспрессируется на содержащих ядро клетках;
- переносимость (менее выраженные системные побочные эффекты);
- результаты исследования LIMT-HDV (фаза I) по применению ПЭГ-ИФН-λ (180 мкг/нед) продемонстрировали снижение уровней РНК HDV в сыворотке в среднем на 2,3 lg на 48-й неделе терапии и отсутствие определяемой РНК HDV через 24 нед после прекращения терапии [25].
Согласно результатам исследования LIMT-HDV (фаза II), при использовании ИФН-λ в сочетании с лонафарнибом и ритонавиром у 96% пациентов наблюдалось снижение РНК HDV >2 lg МЕ/мл, у 58% – достижение УВО через 24 нед [26, 27].
Блокатор входа HDV в клетку
Булевиртид (BLV) – первый синтезированный ингибитор проникновения в гепатоциты вирусных частиц, в состав которых входит поверхностный белок HBV. Он представляет собой миристилированный пептид длиной 47 аминокислот, полученный из оптимизированной консенсусной последовательности preS1-домена L-белка HBV, который эффективно блокирует hNTCP, рецептор HDV/HBV на поверхности гепатоцита. Способность препарата в очень низких концентрациях полностью предотвращать проникновение вируса показана in vitro и in vivo [28].
Предотвращение входа HBV и HDV в клетку приводит к снижению следующих параметров:
- числа инфицированных гепатоцитов;
- концентрации РНК HDV в печени и крови;
- цитолиза (нормализация уровня АЛТ);
- воспаления и фиброза [29].
К концу 2011 г. были завершены доклинические исследования in vitro и in vivo безопасности BLV и выполнена оценка его противовирусной эффективности [30, 31]. Основные результаты законченных и проводимых в настоящее время клинических исследований препарата представлены в таблице 1.
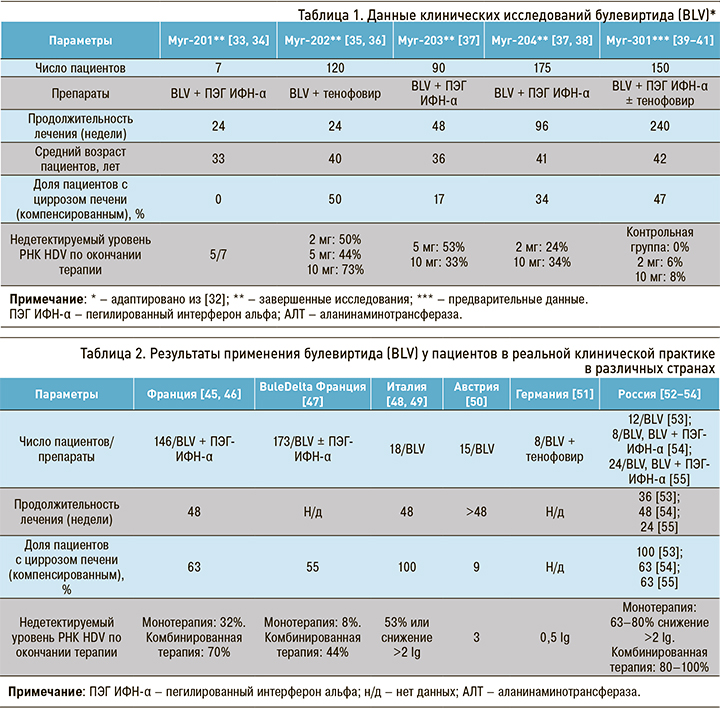
Хорошая безопасность, постоянная противовирусная эффективность и быстрая нормализация уровня АЛТ во время терапии, выявленные в клинических исследования, послужили основанием тому, что в июле 2020 г. было зарегистрировано разрешение на продажу BLV (в дозе 2 мг) в РФ. BLV под коммерческим названием Hepcludex® получил одобрение Европейского медицинского агентства в июле 2020 г. в виде флаконов по 2 мг для ежедневного подкожного введения [42].
Важно отметить, что в этих исследованиях наряду с прочим представлены результаты оценки безопасности применения BLV у пациентов с ЦП. Частота и выраженность НЯ у больных циррозом оказались сравнимыми с таковыми в общей популяции пациентов как при монотерапии, так и двойной терапии [37].
Определенным ограничением приведенных исследований были относительно небольшие когорты пациентов, различия в дизайне исследований, не позволяющие провести метаанализ на данном этапе, недостаточность данных для оценки гистологического ответа.
Во всех исследованиях отмечена безопасность BLV. Он вызывал повышение концентрации желчных кислот в сыворотке крови у всех пациентов, получавших дозу препарата, которое оставалось клинически бессимптомным (не зарегистрировано ни одного случая зуда) и быстро возвращалось к норме после окончания терапии [43], хотя в одном случае наблюдалась реакция гиперчувствительности [44].
Недавно авторами из нескольких стран были опубликованы наблюдения из клинической практики, которые в целом подтверждают результаты клинических испытаний препарата. Соответствующие данные представлены в таблице 2.
В России в 2022–2023 гг. появились первые сообщения по накопленному опыту противовирусной терапии в реальной клинической практике [52–54]. Авторы ставили своей задачей изучение эффективности и безопасности BLV у пациентов как с ХГD, так и с ЦП HDV-этиологии. В результате проведенных исследований показано, что при монотерапии длительностью 24–48 нед у более 60% пациентов отмечается снижение уровня РНК HDV более чем на 2,0 lg копий/мл. Уровень АЛТ снизился до нормальных значений у 75% больных. В исследованиях отмечалась хорошая переносимость препарата – отсутствие тяжелых НЯ (случаев отмены BLV и ухудшения самочувствия).
Добавим, что во всех представленных выше наблюдениях комбинация снижения вирусной нагрузки >2 lg и нормализации АЛТ (комбинированный ответ) использовался в качестве первичной конечной точки. Использование этой конечной точки подразумевает, что у многих пациентов все еще остается определяемая вирусная РНК, а влияние неполной вирусной супрессии на дальнейшее развитие ХГD остается неизвестным. Кроме того, такой подход проблематичен у пациентов с низкой исходной вирусной нагрузкой (≤104 lg копий/мл) и низким/нормальным уровнем АЛТ [50]. АЛТ не является точным маркером заболевания печени, так как у пациентов с прогрессирующим хронической патологией печени этот показатель может быть в пределах нормы [55, 56].
Таким образом, подводя итоги анализа полученных результатов клинических исследований и данных реальной клинической практики, становится очевидным, что количественное определение РНК HDV служит единственным надежным маркером репликации HDV и должно быть неотъемлемой частью мониторинга лечения пациентов с ХГD. За последнее десятилетие было разработано несколько коммерческих и лабораторных тестов для количественного определения вирусной нагрузки HDV. В исследовании Le Gal F. et al. было показано, что более половины из них не смогли выявить или правильно определить количественно часть тестовых образцов РНК HDV разных генотипов вируса [57]; это подчеркивает отсутствие эффективных инструментов для рутинного мониторинга уровня РНК HDV у пациентов, находящихся на терапии. К сожалению, практически ни в одной статье, проанализированной в данном обзоре, не было информации о тест-системах, использованных для количественного определения вирусной нагрузки при применении BLV. Необходимо отметить, что в России в настоящее время не существует зарегистрированных тест-систем для количественного определения вирусной нагрузки HDV, притом что наличие стандартизированных тест-систем для количественного определения РНК HDV – необходимое условие для точной оценки эффективности новых методов лечения HDV в крупных многоцентровых исследованиях. Это важный шаг на пути к выработке согласованных рекомендаций по ведению пациентов, инфицированных HDV.
Важным общим результатом проведенных исследований стало выявление повышения уровня желчных кислот в сыворотке со значительным разбросом значений у отдельных пациентов во время терапии, что, как и ожидалось, является следствием ингибирования NTCP. Определение желчных кислот в сыворотке крови может быть полезным для контроля приверженности к терапии. Продолжаются исследования по изучению влияния лечения на фиброз печени и прямые конечные точки, такие как неблагоприятные исходы инфекции и смертность. Помимо противовирусного ответа, BLV может оказывать гепатопротекторное действие, поскольку ингибирование NTCP вызывает снижение рециркуляции желчных кислот. Изменение транспорта желчных кислот может быть важным модулятором фиброза печени, что предотвращает прогрессирование заболевания [58]. Таким образом, ингибирование NTCP может быть полезным при некоторых формах холестаза [59] и способно оказывать противовоспалительное действие [60].
Ограничения этих исследований включают отсутствие предварительно определенного протокола лечения и отсутствие данных об отдаленных результатах после прекращения терапии, поскольку большинство пациентов продолжают лечение BLV.
ИНГИБИТОРЫ ПРЕНИЛИРОВАНИЯ КАПСИДНОГО БЕЛКА HDV
В настоящее время исследуется группа лекарственных средств, влияющих на процессы посттрансляционной модификации капсидного белка (большого антигена, или L-HDAg) HDV, в частности процесса его пренилирования. Представитель этой группы препаратов лонафарниб относится к ингибиторам фарнезилтрансферазы – фермента клетки, позволяющего изопренилированному L-HDAg присоединиться к эндоплазматической сети [61]. Снижение уровней РНК HDV в сыворотке является следствием подавления сборки новых вирионов HDV, но не приводит к снижению числа инфицированных гепатоцитов. Одним из эффектов лонафарниба может стать ускорение оборота инфицированных гепатоцитов за счет прямого цитотоксического действия накапливающихся репликативных промежуточных продуктов HDV или усиленного иммуноопосредованного уничтожения инфицированных клеток [62–65]. Первоначально разработанный в качестве противоракового препарата лонафарниб был исследован в нескольких клинических испытаниях (фазы I и II) с декабря 2011 г. по июнь 2016 г. Пациенты (n=14) в течение 4 нед получали 100 или 200 мг лонафарниба в сутки. Снижение вирусной нагрузки HDV составило 0,75 и 1,25 lg МЕ/мл в зависимости от дозы препарата. Значимым побочным эффектом стали выраженные и дозозависимые желудочно-кишечные осложнения [66].
В исследованиях III фазы введение ритонавира (фармакокинетического усилителя) позволило форсировать дальнейшее увеличение противовирусного действия лонафарниба и снизить частоту нежелательных побочных эффектов [65]. В целом исследования свидетельствуют, что лечение лонафарнибом вызывает снижение уровня РНК HDV и нормализацию ферментов печени у пациентов с HDV-инфекцией. На сегодняшний день исследуются комбинации этого препарата с ПЭГ-ИФН-α или ИФН-λ [67]. Исследование, в котором изучалось применение ПЭГ-ИФН-λ в комбинации с лонафарнибом в течение 24 нед, показало негативацию по РНК HDV или снижение концентрации РНК HDV ниже нижнего предела количественного определения у 11 из 22 пациентов (50%) в конце лечения. Во время наблюдения рецидив произошел у 6 пациентов [68].
ПОЛИМЕРЫ НУКЛЕИНОВЫХ КИСЛОТ
Еще одной группой соединений, потенциально обладающей активностью в отношении HDV, являются полимеры нуклеиновых кислот (NAP). NAP представляют собой разнообразную группу олигонуклеотидов, которые были описаны как агенты с широким спектром противовирусной активности, включая ингибирование ВИЧ, вируса простого герпеса (HSV) или вируса лимфоцитарного хориоменингита (LCMV) [69]. Первоначально предполагавшийся механизм действия NAP заключался в их связывании с молекулами, участвующими в обеспечении проникновения вируса (например, гепарансульфат-протеогликаны). Blanchet M. et al. показали, что один из NAP, REP 2139, блокирует сборку субвирусных частиц в гепатоцитах, несущих кзкДНК или интегрированную ДНК HBV, что приводит к снижению секреции HBsAg и его содержания в клетках печени [70, 71].
NAP были протестированы на экспериментальной модели инфекции вируса гепатита B уток (DHBV). Было установлено, что одно соединение, REP2006, влияет преимущественно на проникновение вируса, тогда как другое, REP2055, подавляет секрецию поверхностного белка DHBV при персистирующей инфекции DHBV [72, 73]. Основываясь на результатах модели DHBV-инфекции, первые два клинических исследования были проведены на пациентах с моноинфекцией HBV [74]. Поскольку результаты этих испытаний показали выраженное снижение уровней HBsAg в сыворотке крови, полимер нуклеиновых кислот REP2139-Ca был протестирован у пациентов с хронической HDV-инфекцией. 12 пациентов с коинфекцией HBV/HDV получали монотерапию 500 мг REP2139-Ca (внутривенно 1 раз/нед) в течение 15 нед с последующей комбинацией 250 мг препарата с 180 мкг ПЭГ-ИФН-α в течение еще 15 нед. Терапия ПЭГ-ИФН-α продолжалась еще 33 нед, а пациенты наблюдались в течение 24 нед. Промежуточные результаты указывали на выраженное снижение в сыворотке крови как РНК HDV, так и уровней HBsAg [75]. В 2021 г. Bazinet M. et al. установили, что достигнутые результаты сохранялись после 3,5 лет наблюдения [76]. Эти обнадеживающие предварительные данные, полученные на небольшой когорте пациентов, должны быть подтверждены в более масштабных исследованиях.
ЗАКЛЮЧЕНИЕ
Дальнейшие разработки новых подходов к терапии ХГD должны учитывать различные механизмы действия лекарственных препаратов и строиться на основе комбинированной терапии. Из представленных данных становится очевидным, что BLV переносится пациентами достаточно хорошо, и его противовирусная эффективность возрастает с увеличением продолжительности лечения. Тем временем лонафарниб демонстрирует более выраженный ранний ВО, но в некоторых случаях наблюдается некоторое снижение его противовирусной эффективности, особенно после 24 нед лечения. Таким образом, возможно использовать повторные курсы лечения на основе лонафарниба и оценивать эффект после повторного его применения.
Следует отметить, что наилучшие результаты при применении новых соединений были получены, когда они применялись в сочетании с ПЭГ-ИФН-α. Возможно, интерфероны и в дальнейшем будут использоваться в качестве основы терапии, но, вероятно, ПЭГ-ИФН-α будет заменен на ПЭГ-ИФН-λ. Тем не менее необходимы разработки безинтерфероновых схем лечения ХГD. Новые исследования должны обязательно учитывать несколько гематологических, биохимических, серологических и вирусологических параметров в качестве потенциальных предикторов ответа для формирования концепции полного излечения.



