ВВЕДЕНИЕ
Сахарный диабет (СД) относится к независимым факторам риска атеросклеротических сердечно-сосудистых заболеваний (АССЗ). Несмотря на многолетний прогресс в понимании патогенеза и лечении СД, его распространенность продолжает расти, что приводит к значительному увеличению бремени заболеваемости и сокращению продолжительности жизни, главным образом за счет сосудистых осложнений [1].
Наряду с контролем уровня глюкозы и коррекцией других факторов кардиоваскулярного риска (дислипидемии, гипертонии, курения), ключевым компонентом тактики ведения пациентов с СД служит антитромботическая терапия (АТТ). В рамках АТТ применяются антиагреганты и пероральные антикоагулянты (ПОАК), цель назначения которых – профилактика атеротромботических и кардиоэмболических событий. За последние десятилетия АТТ значительно изменилась: увеличился как ассортимент доступных лекарств, так и понимание того, как лучше всего их использовать [2].
У пациентов с СД АТТ представляет определенные сложности ввиду особенностей процессов тромбообразования. При СД в организме создается протромботическая среда, обусловленная множественными патологическими процессами. Гипергликемия, хроническое воспаление, оксидативный стресс и связанные с ними метаболические состояния вызывают эндотелиальную дисфункцию, что также влечет за собой активацию тромбоцитов [3].
На этом уровне действует целый ряд механизмов, повышающих реактивность тромбоцитов. Во-первых, гипергликемия сопряжена с более высокой экспрессией рецепторов тромбоцитов, таких как гликопротеин (GP)Ibα, GPIIb/IIIa и P2Y12. Во-вторых, повышенное гликирование приводит к снижению текучести мембран тромбоцитов, а оксидативный стресс увеличивает выработку тромбоксана А2 и F2-изопростанов, которые, в свою очередь, активируют рецепторы тромбоксана и усиливают активацию тромбоцитов [4–8]. Кроме того, в условиях снижения чувствительности тканей к инсулину происходит снижение восприимчивости к антиагрегантным агентам – простациклину и оксиду азота, что также вызывает гиперактивность тромбоцитов [9–11]
При СД наблюдается ускоренный оборот тромбоцитов, о чем свидетельствует высвобождение более реактивных сетчатых тромбоцитов, которые демонстрируют сниженный ответ на антиагреганты [12–14].
Сопутствующие диабету метаболические состояния в виде ожирения, дислипидемии и системного воспаления также повышают риск тромбоза [15, 16]. Циркулирующие воспалительные молекулы (фактор некроза опухоли-альфа, интерлейкины 1 и 6, растворимый лиганд CD40) не только усиливают реактивность тромбоцитов, но и повышают общий воспалительный фон, способствуя гиперкоагуляции [17, 18].
При СД наблюдаются некоторые протромботические изменения в коагуляционно-фибринолитической системе: возрастают уровни тканевого фактора, протромбина, фактора VII и фибриногена в сочетании с нарушением антикоагулянтной и фибринолитической активности [19, 20]. Учитывая формирование такой протромботической среды, пациентам с СД необходима АТТ для снижения частоты больших неблагоприятных сердечно-сосудистых событий (MACE). Однако важно понимать, что это может приводить к увеличению кровотечений. Уравновешивание рисков и пользы АТТ имеет решающее значение для интерпретации данных клинических исследований и разработки рекомендаций по лечению, в том числе у пациентов с СД [21].
АНТИТРОМБОТИЧЕСКАЯ ТЕРАПИЯ У ПАЦИЕНТОВ С САХАРНЫМ ДИАБЕТОМ БЕЗ ПРИЗНАКОВ СЕРДЕЧНО-СОСУДИСТЫХ ЗАБОЛЕВАНИЙ
Как нами уже было отмечено, СД сам по себе выступает предрасполагающим фактором к нарушениям в системе гемостаза, которые повышают риск атеротромбоза и тромботических осложнений. Учитывая это обстоятельство, важным представляется обсуждение вопроса о необходимости и безопасности превентивной АТТ у пациентов с СД даже при отсутствии у них симптомов и/или анамнестических данных, позволяющих предположить наличие АССЗ, в том числе острого (ОКС) или хронического коронарного синдрома (ХКС), поражения периферических артерий, MACE.
Метаанализ Baigent C. et al., проведенный на основе данных 95 000 пациентов со средним сердечно-сосудистым риском (0,57% MACE/год) из шести рандомизированных контролируемых исследований (РКИ), показал, что низкие дозы ацетилсалициловой кислоты (АСК) значительно снижают риск MACE по сравнению с контрольной группой (абсолютное снижение риска на 0,06% в год, p=0,0001), одновременно увеличивая количество больших экстракраниальных кровотечений (0,10 против 0,07% в год; абсолютное увеличение риска на 0,03% в год; p <0,0001). В подгруппе пациентов с СД (n=3818, 4% общей выборки) соотношение пользы и безопасности было аналогичным [22].
Исследование ASCEND – крупнейшее плацебо-контролируемое РКИ с тестированием низких доз АСК у пациентов с СД 1-го или 2-го типа (n=15 480) без явных ССЗ – продемонстрировало схожую с предыдущим метаанализом пользу от применения этого антиагреганта. За 7,4 года прием АСК по сравнению с плацебо значительно уменьшил количество серьезных сосудистых осложнений (8,5 против 9,6% соответственно), но в то же время увеличил риск кровотечений 3–5-го типа по шкале BARC (4,1 и 3,2%, соответственно). Важно отметить, что увеличение числа геморрагий в группе АСК отмечалось в основном за счет желудочно-кишечных кровотечений, без существенных различий в количестве смертельных, внутричерепных и глазных кровотечений [23].
У части пациентов в исследовании ASCEND эффективность АСК была продемонстрирована на фоне приема статинов (75% пациентов) и/или антигипертензивных препаратов (60%). Недавний метаанализ 18 162 пациентов с множественными факторами риска ССЗ (риск 1,7%/год), но без ССЗ в анамнезе также позволил установить значительное преимущество низких доз АСК в комбинации с препаратами, снижающими уровень липидов и артериальное давление, что наблюдалось и в подгруппе пациентов с СД [24].
Последующий анализ исследования ASCEND продолжительностью 9,2 года, посвященный изучению влияния АСК на развитие у пациентов деменции и когнитивных нарушений, продемонстрировал отсутствие соответствующих негативных эффектов АСК и даже тенденцию к снижению риска деменции на фоне приема этого препарата, что также было подтверждено метаанализом трех крупных РКИ по первичной профилактике [25].
На основании представленных данных авторы рекомендаций Европейского кардиологического общества (ESC) считают допустимым назначение низких доз АСК (75–100 мг 1 раз/сут) пациентам с СД без признаков ССЗ и факта проведения реваскуляризации в анамнезе для предупреждения первого MACE в отсутствие противопоказаний в виде желудочно-кишечного кровотечения или язвенной болезни в течение предыдущих 6 мес, активных заболеваний печени (цирроза, активного гепатита) или аллергии на АСК в анамнезе. При этом в рекомендациях подчеркивается, что назначение АСК может иметь значительно бóльшую эффективность и целесообразность у пациентов с уже имеющимися бессимптомными АССЗ (в том числе подтвержденными методами визуализации). В качестве биомаркера для выявления бессимптомных пациентов с повышенным риском ССЗ рассматривается оценка уровня кальциноза коронарных артерий – перспективный неинвазивный метод скрининга, эффективность которого показана на обширных проспективных данных и продолжает исследоваться [26].
Таким образом, пациентам с СД без признаков ССЗ для профилактики тромботических осложнений может быть назначена АСК в низких дозах (75–100 мг), однако при этом требуется индивидуальная оценка соотношения риска и пользы для каждого пациента. Большей потенциальной эффективности назначения АСК в качестве АТТ следует ожидать у пациентов с подозрением на наличие бессимптомных АССЗ (по данным методов визуализации, в том числе с признаками кальциноза коронарных артерий).
АНТИТРОМБОТИЧЕСКАЯ ТЕРАПИЯ У ПАЦИЕНТОВ С САХАРНЫМ ДИАБЕТОМ И ХРОНИЧЕСКИМ КОРОНАРНЫМ СИНДРОМОМ, КОТОРЫМ НЕ ТРЕБУЕТСЯ ДЛИТЕЛЬНАЯ АНТИКОАГУЛЯНТНАЯ ТЕРАПИЯ
Группа пациентов с ХКС объединяет несколько подгрупп: больных со стабильной, вазоспастической или микроваскулярной стенокардией, недавно возникшей сердечной недостаточностью с подозрением на поражение коронарных артерий, стабильным течением заболевания, связанного с поражением коронарных артерий, пациентов, которым более года назад был установлен диагноз или проведена реваскуляризация, а также бессимптомных больных с подтвержденным атеросклерозом коронарных артерий. Часть таких пациентов имеют показания к назначению ПОАК, тогда как у тех пациентов, которые не получают терапию этими препаратами, необходимо решение вопроса о назначении другой АТТ.
Пациенты с СД и подтвержденными значимыми ССЗ или фактом реваскуляризации в анамнезе имеют очень высокий сердечно-сосудистый риск и должны получать АСК в низких дозах (75–100 мг/ сут) для профилактики тромботический осложнений.
У пациентов с непереносимостью АСК альтернативой ей может служить клопидогрел, который также следует добавлять к низким дозам АСК (клопидогрел 75 мг + АСК 75–100 мг/сут) в рамках двойной антитромбоцитарной терапии (ДАТТ) у больных хронической сердечной недостаточностью, перенесших чрескожное коронарное вмешательство (ЧКВ). Исследования ADAPTABLE и CURRENT-OASIS 7 показали сопоставимую эффективность низких (75–100 мг/сут) и высоких (300–325 мг/сут) доз АСК как при ХКС, так и ОКС [27, 28].
В работе THEMIS исследовалась эффективность и безопасность добавления к АСК (75–100 мг/сут) ингибитора P2Y12 тикагрелора (60 мг внутривенно) по сравнению с плацебо у 19 220 пациентов с СД и перенесенным в анамнезе ЧКВ, аортокоронарным шунтированием или документально подтвержденным стенозом (≥50% по крайней мере в одной коронарной артерии) в отсутствие предшествующего инфаркта миокарда или инсульта. Результаты показали неудовлетворительный профиль безопасности и эффективности такой терапии у наблюдавшихся пациентов [29].
Исследование COMPASS, включившее 27 395 пациентов с хроническими ССЗ (перенесенным инфарктом миокарда, симптомными заболеваниями периферических артерий), продемонстрировало превосходство комбинации низких доз АСК с очень низкими дозами ривароксабана (2,5 мг внутримышечно) в предотвращении MACE по сравнению с группой АСК и плацебо (4,1 против 5,4% соответственно). Международное общество по тромбозу и гемостазу определило увеличение частоты больших кровотечений в группе пациентов, принимавших ривароксабан и АСК, относительно лиц на монотерапии аспирином (3,1 и 1,9% соответственно), при этом значимого увеличения смертельных и внутричерепных кровотечений не отмечалось. Профиль пользы и риска в подгруппе больных СД (38% всех пациентов) был аналогичен основной выборке, исходя из чего, авторы рекомендуют рассмотреть возможность добавления ривароксабана в очень низких дозах к АСК в низких дозах для долгосрочной профилактики серьезных сосудистых осложнений у пациентов с СД и ХКС или симптомным поражением периферических артерий в отсутствие высокого риска кровотечений. Важно добавить, что, поскольку данные о применении АСК в сочетании с очень низкими дозами ривароксабана исследовались в течение 47 мес, решение о последующем продолжении этой терапии должно приниматься индивидуально и с регулярной оценкой рисков тромботических осложнений и кровотечений [30].
Таким образом, всем пациентам с СД и ХКС, не нуждающимся в назначении длительной антикоагулянтной терапии, согласно последним рекомендациям, в качестве АТТ следует назначать АСК в низких дозах (а при ее непереносимости клопидогрел). При этом у пациентов после ЧКВ рекомендовано назначение в течение 6 мес ДАТТ в виде комбинации АСК с клопидогрелом. Кроме того, у больных с СД и ХКС или атеросклерозом периферических артерий рекомендовано рассмотреть возможность добавления к терапии АСК ривароксабана в очень низких дозах. Назначение тикагрелола таким пациентам не рекомендуется.
АНТИТРОМБОТИЧЕСКАЯ ТЕРАПИЯ У ПАЦИЕНТОВ С САХАРНЫМ ДИАБЕТОМ И ХРОНИЧЕСКИМ КОРОНАРНЫМ СИНДРОМОМ, НУЖДАЮЩИХСЯ В ДЛИТЕЛЬНОЙ АНТИКОАГУЛЯНТНОЙ ТЕРАПИИ
У пациентов с СД и ХКС с показаниями к длительной антикоагулянтной терапии (больные с наличием фибрилляции предсердий или проходящие ЧКВ по поводу ХКС) в настоящее время сравнивается целесообразность назначения двойной (ПОАК + клопидогрел) или тройной (ПОАК + АСК + клопидогрел) АТТ, причем однозначных выводов на этот счет пока не сделано. Имеющиеся исследования ограничены невысокой продолжительностью наблюдения и небольшими выборками, поэтому недостаточно эффективны для оценки эффективности ДАТТ и безопасности тройной терапии у пациентов с СД. Результаты двух метаанализов свидетельствуют о большей эффективности тройной АТТ в отношении снижения риска тромботических осложнений [31, 32].
Таким образом, исходя из отсутствия достоверных доказательств эффективности и безопасности, рекомендовано осторожно и систематически оценивать индивидуальное соотношение пользы и риска у каждого отдельного пациента с СД и ХКС.
У пациентов с СД и фибрилляцией предсердий, получающих АТТ и имеющих показания к антикоагулянтной терапии, при отсутствии противопоказаний рекомендовано предпочесть ПОАК варфарину.
Пациентам с СД и ХКС, которым проводится имплантация коронарного стента и у которых есть показания к антикоагулянтной терапии, рекомендуется тройная АТТ (ПОАК + АСК + клопидогрел) в течение как минимум 1 нед (при этом возможно продление терапии до 1 и даже до 3 мес с учетом индивидуальной оценки профиля пользы и риска) с последующим переходом на ДАТТ (ПОАК + АСК или ПОАК + клопидогрел).
АНТИТРОМБОТИЧЕСКАЯ ТЕРАПИЯ У ПАЦИЕНТОВ С САХАРНЫМ ДИАБЕТОМ И ОСТРЫМ КОРОНАРНЫМ СИНДРОМОМ
У больных с ОКС, перенесших ЧКВ, в ряде исследований ДАТТ в течение 12 мес с применением низких доз АСК и прасугрела или тикагрелора превосходила ДАТТ АСК + клопидогрел в подгруппе пациентов с СД [33, 34]. Возможно, это обусловлено пониженным образованием активного метаболита клопидогрела при СД [35].
В прямом сравнительном исследовании ISAR-REACT 5 было рандомизировано 4018 пациентов с ОКС на группы приема прасугрела или тикагрелора в дополнении к терапии АСК. Прасугрел превосходил тикагрелор в снижении МАСЕ без увеличения массивных кровотечений с аналогичными эффектами в подгруппе пациентов с диабетом (n=892; 22%) [36]. Таким образом, низкие дозы АСК в сочетании с прасугрелом или тикагрелором предпочтительнее ДАТТ с клопидогрелом у пациентов с СД и ОКС в отсутствие очень высокого риска кровотечения [37].
На основании данных исследования PEGASUS-TIMI 54, доказавшего эффективность длительной терапии тикагрелором в дозе 60 мг, метаанализа Khan et al. и исследования DAPT, продемонстрировавших, что пролонгированная ДАТТ снижает частоту MACE и цереброваскулярных событий, но в то же время увеличивает количество кровотечений, следует рассматривать продление ДАТТ более 12 мес (на срок до 36 мес) у пациентов с СД, которые переносят ДАТТ без больших кровотечений [36–38]. В настоящее время нет достаточно данных по безопасности и эффективности ДАТТ с уменьшенной дозой тикагрелора в течение более 3 лет. В связи с отсутствием достоверных доказательств эффективности и безопасности сокращение или деэскалация ДАТТ не рекомендуются рутинно пациентам с СД в течение 12 мес после ОКС [39].
На рисунке обобщены рекомендации по АТТ для пациентов с СД и ОКС или ХКС, подвергшихся реваскуляризации миокарда.
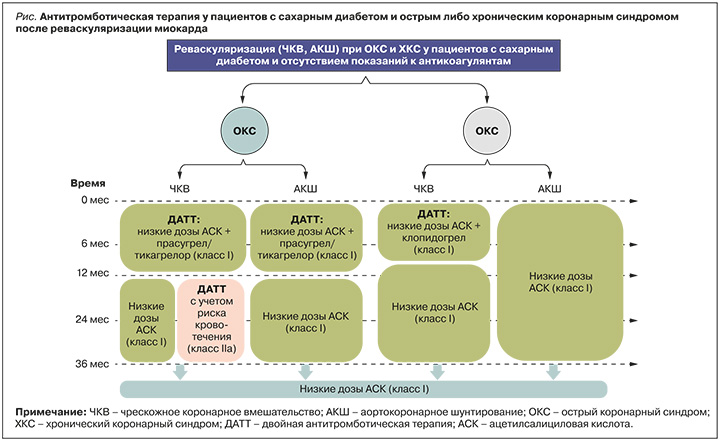
АНТИТРОМБОТИЧЕСКАЯ ТЕРАПИЯ У ПАЦИЕНТОВ С САХАРНЫМ ДИАБЕТОМ И ПЕРИФЕРИЧЕСКИМ АТЕРОСКЛЕРОЗОМ
В недавнем исследовании было установлено, что комбинация низких доз АСК и ривароксабана в дозе 2,5 мг внутривенно снижает риск развития MACE и основных нежелательных явлений со стороны конечностей, включая ампутацию у мужчин, по сравнению с АСК и плацебо, особенно при поражении периферических артерий [40]. В группе приема ривароксабана увеличилось общее число серьезных кровотечений, однако смертельных или критических кровотечений из органов выявлено не было.
АНТИТРОМБОТИЧЕСКАЯ ТЕРАПИЯ У ПАЦИЕНТОВ С САХАРНЫМ ДИАБЕТОМ 1-ГО ТИПА
Применение антитромбоцитарных препаратов может быть рассмотрено у лиц с СД 1-го типа без симптомов ССЗ при наличии по крайней мере одного дополнительный фактора кардиоваскулярного риска [41].
ПРОФИЛАКТИКА ЖЕЛУДОЧНО-КИШЕЧНЫХ КРОВОТЕЧЕНИЙ ПРИ АНТИТРОМБОТИЧЕСКОЙ ТЕРАПИИ
Профилактика кровотечений на фоне приема АТТ остается крайне актуальной проблемой. Во многих РКИ показано, что прием ингибиторов протонной помпы предотвращает развитие желудочно-кишечных кровотечений как у пациентов с СД, так и без него. При комбинированной АТТ для предотвращения желудочно-кишечных кровотечений рекомендуются ингибиторы протонной помпы [42].
В случае использования единственного антитромбоцитарного препарата также следует рассмотреть возможность применения гастропротекторов для предотвращения желудочно-кишечного кровотечения, учитывая риск кровотечения у конкретного пациента [43-45].
НАПРАВЛЕНИЯ БУДУЩИХ ИССЛЕДОВАНИЙ
Несмотря на то что появляется все больше данных об эффективности и безопасности АТТ при СД, исследований, спланированных непосредственно для изучения этой когорты пациентов, все еще не много. Интерес представляет тактика назначения АТТ с целью первичной и вторичной профилактики MACE при СД 1-го и 2-го типа. Для больных диабетом характерна высокая гетерогенность уровня сердечно-сосудистого риска, который зависит от наличия коморбидной патологии и продолжительности заболевания у конкретного пациента. В связи с этим до сих пор не существует однозначных рекомендаций о порядке назначения им АСК для первичной кардиоваскулярной профилактики. Вероятно, дать ответы на данные вопросы могли бы РКИ с участием пациентов с СД, не переносивших MACE, но имеющих микрососудистые поражения органов-мишеней. Такие патологии являются независимыми предикторами МАСЕ, в связи с чем могут повлиять на тактику назначения АТТ для первичной профилактики. Также требует уточнения тактика применения АТТ у пациентов с СД и ОКС. Остается неясным, можно ли сократить 12-месячный период ДАТТ.
Еще одно важнейшее направление будущих исследований – поиск биомаркеров, способных повысить точность определения риска тромботических осложнений и кровотечений. До настоящего времени риск кровотечений у пациентов с СД изучался преимущественно в подгруппах крупных РКИ, которые не были спланированы для этих целей. Более того, в будущих РКИ по оценке эффективности и безопасности АТТ у пациентов с СД необходимо использовать универсальные классификации кровотечений, чтобы сделать профиль пользы и риска моно- или комбинированной терапии сопоставимым в различных исследованиях.
ЗАКЛЮЧЕНИЕ
Пациенты с СД представляют собой гетерогенную группу коморбидных пациентов с повышенным риском МАСЕ. Важнейшим звеном медикаментозной профилактики осложнений у пациентов с СД служит АТТ. Новые европейские рекомендации по диагностике и лечению СД проливают свет на множество ранее не изученных клинических ситуаций, в которых пациентам с этим заболеванием требуется назначение АТТ, в частности при сочетании СД и таких патологий, как ХКС, ОКС, фибрилляция предсердий, сердечная недостаточность. Появляется все больше данных о риске кровотечений и тактике их профилактики при СД. Тем не менее, требуется более глубокое понимание метаболических процессов при СД, которые могут влиять на эффективность и безопасность АТТ у этой группы пациентов. Необходим поиск новых биохимических маркеров риска кровотечений и тромботических осложнений, а также планирование новых РКИ, главной целью которых станет изучение АТТ у больных с СД в различных клинических ситуациях.



