Лечение заболеваний печени во время беременности, с которыми часто сталкиваются в своей повседневной практике врачи разных специальностей, является довольно сложной проблемой и требует многопрофильного подхода. Физиологические и анатомические изменения во время беременности, сложное взаимодействие между матерью и плодом и редкость заболевания печени во время самой беременности – вот лишь некоторые из многих задач, которые приходится решать практическому врачу при лечении таких заболеваний. Кроме того, потенциальные последствия для плода и матери должны учитываться при проведении различных диагностических и терапевтических мероприятий.
Тяжелые формы заболеваний печени приводят к высокой материнской и перинатальной заболеваемости и смертности [1]. Для выбора рациональной терапевтической тактики чрезвычайно важна быстрая дифференциальная диагностика заболеваний, связанных с беременностью, и заболеваний, с беременностью не связанных, но начавшихся во время беременности или возникших до нее (табл. 1).
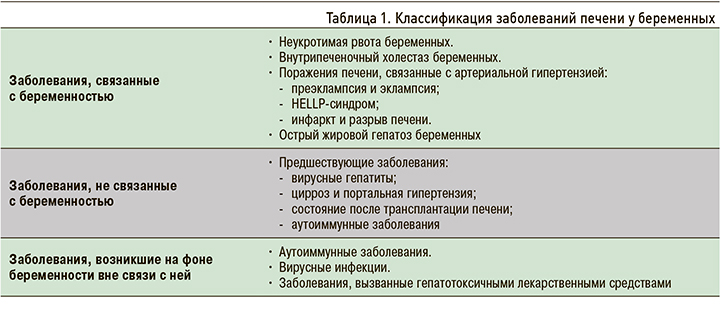
В настоящей статье рассмотрены болезни, связанные с беременностью, при этом особое внимание уделено внутрипеченочному холестазу беременных как наиболее распространенному заболеванию печени при беременности [2, 3].
ПОРАЖЕНИЯ ПЕЧЕНИ, СВЯЗАННЫЕ С БЕРЕМЕННОСТЬЮ
Специфичные для беременности поражения печени возникают в зависимости от сроков беременности и, как правило, регрессируют после родоразрешения. К числу таких заболеваний относятся неукротимая рвота беременных, острая жировая дистрофия печени беременных, HELLP-синдром, внутрипеченочный холестаз беременных.
Неукротимая рвота беременных (НРБ) – связанная с беременностью патология, которая возникает в I триместре и характеризуется тошнотой и рвотой тяжелой степени, приводящей к недостаточности питания, потере массы тела, дегидратации, электролитному дисбалансу, кетацидозу и часто повышению активности трансаминаз. Как правило, НРБ дебютирует в начале I триместра и обычно продолжается до 20 нед беременности. Частота НРБ составляет 1–20 случаев на 1000 беременностей.
К факторам риска развития этого заболевания относят НРБ в анамнезе, гипертиреоз, психические расстройства, сахарный диабет, высокий индекс массы тела (ИМТ), плод женского пола, инфекцию Helicobacter pylori (требует дальнейшего изучения), аномалии эмбриона (триплоидия, трисомия 21), многоплодную беременность [3, 4].
Острая жировая дистрофия печени беременных (ОЖДПБ) – жизнеугрожающее для матери и плода осложнение, возникающее во второй половине беременности (чаще в III триместре), иног-да в послеродовом периоде, характеризующееся микровезикулярным стеатозом гепатоцитов вследствие нарушения метаболизма жирных кислот и приводящее к острой печеночной недостаточности. Частота ОЖДБП: 1 случай на 10 000–15 000 беременностей.
Выделяют 3 стадии ОЖДБП:
- преджелтушную;
- желтушную;
- печеночно-почечной недостаточности.
Факторами риска ОЖДПБ являются анамнез нарушения окисления жирных кислот и синдрома Рейе у детей, старший возраст беременных, многоплодная беременность, преэклампсия, плод мужского пола, низкий ИМТ, прием нестероидных противовоспалительных препаратов [3, 4].
HELLP-синдром – жизнеугрожающее для матери и плода осложнение, возникающее во II–III триместрах беременности и в послеродовом периоде, характеризующееся гемолизом, повышением активности печеночных ферментов, уменьшением количества тромбоцитов и приводящее к развитию острой печеночной недостаточности, полиорганной недостаточности, ДВС-синдрому, разрывам печени и гематомам различной локализации. Аббревиатура HELLP включает Нemolysis (гемолиз), Еlevated Liverenzymes (повышение активности печеночных ферментов), Low Plateles (уменьшение количества тромбоцитов). Частота HELLP: 1–6 случаев на 1000 беременных.
Факторы риска HELLP-синдрома – первородящие пациентки старшего возраста, наличие преэклампсии (ПЭ), многоплодной беременности, многоводия, семейного анамнеза ПЭ, сахарного диабета, артериальной гипертонии [3, 4].
Внутрипеченочный холестаз беременных (ВХБ) – наиболее распространенное из связанных с беременностью поражений печени. Это обратимая форма холестаза, для которой характерны зуд и повышение уровня желчных кислот в сыворотке крови. ВХБ спонтанно разрешается в первые 6 нед после родов, но часто рецидивирует при последующих беременностях. Распространенность ВХБ колеблется от 0,7 до 5% [1].
Недостаточность выделения желчи, обусловленная нарушением ее выработки печеночными клетками (гепатоцитами) или прекращением тока желчи по желчным протокам вплоть до дуоденального сосочка (рис. 1), характерна для холестатических заболеваний печени.

Желчные кислоты синтезируются гепатоцитами и затем секретируются в желчевыводящие пути с помощью специализированных транспортеров канальцевых мембран гепатоцитов. Канальцевая желчь выделяется в билиарный тракт и модифицируется выстилающими его эпителиальными клетками – холангиоцитами. Затем желчь попадает в двенадцатиперстную кишку и проксимальную тонкую кишку, в которых метаболизируется кишечными бактериями. Приблизительно 95% желчных кислот реабсорбируются в подвздошной кишке и попадают в воротную вену для рециркуляции обратно в печень через энтерогепатическую циркуляцию. Попав в синусоиды печени, желчные кислоты поглощаются гепатоцитами и секретируются обратно в желчь. Фракция (неконъюгированных) желчных кислот в билиарном тракте поглощается холангиоцитами на апикальной мембране (т.е. до достижения тонкой кишки) и возвращается в синусоиды печени через желчно-печеночный шунт. При холестазе наблюдаются уменьшение канальцевого тока желчи, печеночной экскреции воды и/или органических анионов (билирубина, желчных кислот), накопление желчи в гепатоцитах и желчевыводящих путях, увеличение уровней компонентов желчи в крови (желчных кислот, липидов, билирубина) [5].
Обычно симптомы ВХБ возникают в III триместре беременности, но иногда и на ранних сроках, например, в 7 нед. ВХБ чаще наблюдается при многоплодной беременности и у женщин, ранее лечившихся от бесплодия.
В этиологии ВХБ основную роль играют генетические, эндокринные и средовые факторы (рис. 2). Обусловленное беременностью повышение уровня эстрогенов и прогестерона может вызывать холестаз при наличии скрытой наследственной предрасположенности [1, 2, 6, 7]. Такие случаи встречаются в III триместре, когда концентрации циркулирующих стероидных гормонов и их метаболитов наиболее высоки.
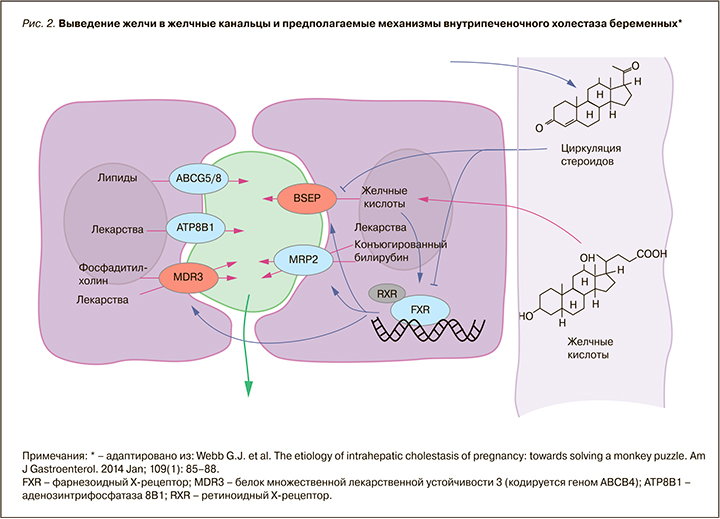
Генетически обусловленная повышенная чувствительность гепатоцитов и билиарных канальцев к половым гормонам, врожденные дефекты синтеза ферментов, ответственных за транспорт компонентов желчи от гепатоцитов в желчные протоки, врожденный дефект синтеза желчных кислот вследствие дефицита ферментов, приводящий к образованию атипичных желчных кислот, не секретируемых транспортными системами канальцевых мембран, составляют основу развития ВХБ [8].
Холестерин транслоцируется третьим АТФ-зависимым ферментом, кодируемым ABCG5/G8, с некоторыми вариациями, связанными с гиперхолестеринемией и желчнокаменной болезнью [10]. Экскреция некоторых лекарств в желчь опосредуется двумя дополнительными АТФазами: ATP8B1 и ABCC2. Внутриклеточные желчные кислоты в гепатоцитах связываются с рецептором FXR. Активированный FXR затем связывается с рецептором RXR и активирует транскрипцию желчных транспортеров. Повышенные уровни циркулирующих стероидов во время беременности непосредственно снижают активность помпы BSEP и других переносчиков. Циркулирующие стероиды также уменьшают транскрипцию канальцевых переносчиков желчных компонентов через подавляющий эффект рецептора FXR. Полиморфизмы в структурах, выделенных красным цветом (см. рис. 2), с высокой степенью достоверности влияют на развитие ВХБ. Структуры, прорисованные синим, предположительно связаны с ВХБ, при этом синие стрелки показывают усиливающие эффекты, а линии без стрелок – снижающие эффекты. Фиолетовые стрелки обозначают движение молекул, зеленые – влияние факторов окружающей среды (в настоящее время недостаточно изучены). В исследовании Reyes H. et al. [11] выявлено, что у женщин с ВХБ достоверно чаще отмечался дефицит селена. Патогенетическое обоснование этого факта авторы нашли в том, что селен служит кофактором ряда ферментных систем печени, многие из которых, по всей видимости, могут играть роль в системе транспорта желчных кислот [2].
Внутрипеченочный холестаз встречается при беременности и как самостоятельное заболевание, и как симптом хронического заболевания гепатобилиарной системы. ВХБ носит семейный характер и развивается у близких родственников (матерей, дочерей и сестер) [8].
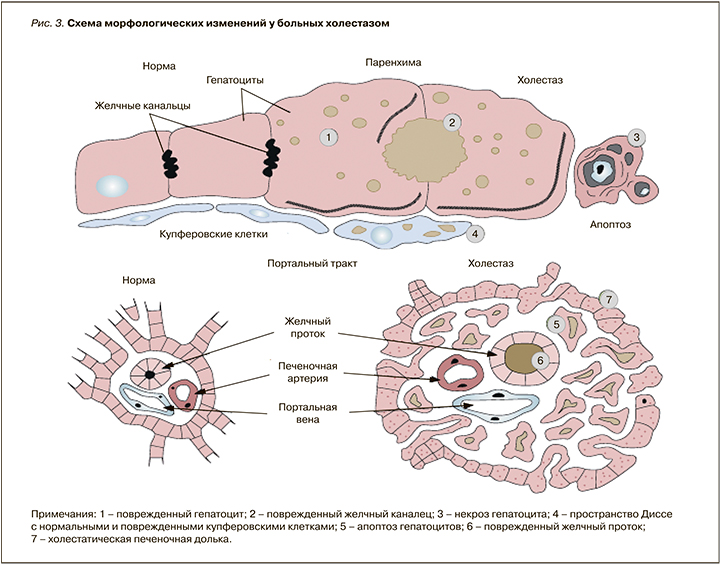
Накопление метаболитов эстрогенов в печени сопровождается нарушением текучести мембран гепатоцитов. Накопление потенциально гепатотоксичных желчных кислот подавляет функцио-нирование Na/К-АТФазы (аденозинтрифосфатазы), в результате чего развивается интралобулярный холестаз. Уплотнение билиарного полюса гепатоцита, снижение текучести каналикулярной мембраны гепатоцитов при сохраненном внутриклеточном транспорте приводит к избыточной концентрации компонентов желчи в гепатоците. Происходит поражение каналикулярного отдела внутрипеченочных желчных протоков. В крови задерживаются компоненты, в норме выделяющиеся в желчь, повышается концентрация желчных кислот. При морфологическом исследовании выявляется накопление желчи в гепатоцитах и желчных путях. Желчные кислоты, накапливаясь при холестазе, могут вызвать некрозы клеток печени и усилить холестаз. Под влиянием желчных кислот повреждаются мембраны митохондрий, что приводит к уменьшению синтеза АТФ в клетке, повышению внутриклеточной секреции кальция, ведущих к повреждению цитоскелета гепатоцита (рис. 3) [12]. Под влиянием желчных кислот повышается внутриклеточная концентрация магния, активируются магнийзависимые трипсиноподобные ядерные протеазы и дегидратация ДНК, вследствие чего активируются процессы апоптоза гепатоцитов, что клинически проявляется синдромом цитолиза [8].
Клиническое значение ВХБ заключается в потенциальном риске для плода (спонтанные или ятрогенные преждевременные роды, асфиксия во время родов, внутриутробная смертность). Так, при ВХБ частота преждевременных родов возрастает в 2,5 раза, риск развития респираторного дистресс-синдрома новорожденных – до 35%, кроме этого, высока вероятность гипоксии плода, задержки его развития [8]. Зуд, особенно сильный по ночам, влияет на качество жизни матери.
КЛИНИЧЕСКИЕ ПРИЗНАКИ ВНУТРИПЕЧЕНОЧНОГО ХОЛЕСТАЗА БЕРЕМЕННЫХ [2]
ВХБ, как правило, дебютирует на 28–30-й неделе беременности появлением кожного зуда, интенсивность которого часто изменчива. Достаточно характерно усиление кожного зуда по ночам. Локализация зуда варьирует от всей поверхности тела до локального зуда ладоней и ступней. Этот симптом может развиваться до появления лабораторных признаков заболевания.
Диспепсические явления (тошнота, рвота, анорексия) и боль в животе возникают относительно редко. Фульминантное течение с нарастанием признаков печеночной недостаточности не характерно для ВХБ и требует поиска других причин заболевания печени, например, наиболее опасного – жирового гепатоза беременных.
При физикальном обследовании целесообразна оценка выраженности желтухи, которая может варьировать от легкой иктеричности склер до интенсивного окрашивания кожных покровов.
В случае обращения за помощью смуглых пациентов или негроидов с целью выявления иктеричности осматривается уздечка языка (склеры могут быть пигментированы), а проявления кожной желтухи оцениваются при осмотре ладоней и стоп. Одна из объективных характеристик кожного зуда – наличие и распространенность расчесов на коже.
Частота возникновения желтухи при ВХБ варьирует от 17 до 75%. Этот симптом обычно развивается через 1–4 нед после появления кожного зуда. Как правило, пациентки обращают внимание на осветление кала. При этом сохраняется относительно хорошее самочувствие, что служит важным дифференциально-диагностическим признаком. Необходимо обращать внимание на наличие гематом, а также прицельно расспрашивать пациентку о наличии кровоточивости десен, носовых и геморроидальных кровотечений, что может свидетельствовать о дефиците витамина К. Значительная гепато- и спленомегалия при физикальном обследовании выявляется чрезвычайно редко.
ДИАГНОСТИКА
Первый шаг в оценке клинической ситуации у женщины с измененными тестами печени на любом сроке беременности включает стандартный диагностический набор:
- выяснение жалоб и анамнеза:
- выявление хронических заболеваний печени, связанных и не связанных с беременностью (гепатиты разной этиологии, цирроз печени, болезнь Вильсона–Коновалова, наследственный гемохроматоз);
- выявление возможных случаев приема гепатотоксических лекарственных препаратов;
- физикальный осмотр и детализацию лабораторных данных в соответствии с клинической картиной [4].
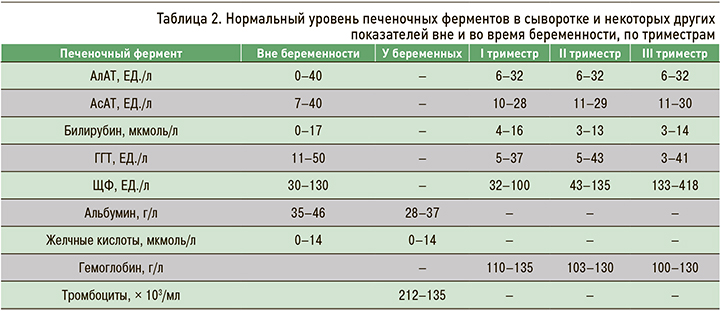
Беременность сопровождается выходящими за стандартные пределы нормы изменениями некоторых биохимических и гематологических показателей (табл. 2). Так, уровень щелочной фосфатазы (ЩФ) в III триместре беременности возрастает за счет ее выработки плацентой и развития костной ткани плода. Происходит повышение уровня α-фетопротеина, который вырабатывается печенью плода. Другие широко используемые в клинической практике биохимические и гематологические показатели (уровень мочевины и гемоглобина, протромбиновое время) не меняются или слегка снижаются вследствие гемодилюции. Повышение во время беременности уровня аминотрансфераз и билирубина и увеличение протромбинового времени свидетельствуют о патологии и требуют диагностического обследования. Для беременности характерно состояние прокоагуляции – повышение уровня факторов свертывания (I, II, V, VII, X и XII) и фибриногена [1].
Основной жалобой при ВХБ бывает зуд, обычно наиболее сильно выраженный на ладонях и подошвах, иногда на других участках кожи или равномерный по всему телу. На коже обнаруживаются экскориации (расчесы), при этом кожная сыпь отсутствует. Порой пациентки отмечают темную окраску мочи и обесцвечивание кала. Характерные для ВХБ изменения биохимических показателей: повышение АлАТ в 1,5–8 раз; повышение концентрации желчных кислот в 1,5–15 раз; концентрация общего билирубина в большинстве случаев не превышает нормы [1].
Концентрация желчных кислот в сыворотке при ВХБ – ценный прогностический показатель, отражающий риск неблагоприятного исхода беременности (спонтанных или индуцированных преждевременных родов, необходимости госпитализации новорожденного, мертворождения) [1, 13]. При концентрации желчных кислот менее 40 мкмоль/л неблагоприятные исходы наступали редко, при превышении этого уровня их частота возрастала. Проведенные исследования [13] не обнаружили зависимости неблагоприятного исхода беременности от уровня аланинаминотрансферазы (АлАТ) и/или аспартатаминотрансферазы (АсАТ), хотя у большинства беременных с ВХБ уровень печеночных аминотрансфераз превышал норму. Концентрация желчных кислот в сыворотке крови подвержена колебаниям и нарастает по мере увеличения срока беременности, поэтому, если результаты в норме, но симптомы ВХБ сохраняются, ее надо контролировать: каждые две недели при сроке беременности менее 34 нед; 1 раз в неделю при сроке беременности более 34 нед. Скрининг свертываемости крови необходимо проводить при наличии клинических признаков жировой мальабсорбции, уровне желчных кислот выше 40 мкмоль/л, быстро повышающихся уровнях желчных кислот или при родах/до планового кесарева сечения в случае аномальных уровней печеночных проб.
Критерии диагноза ВХБ: кожный зуд в сочетании с повышением концентрации желчных кислот в сыворотке при разрешении обоих симптомов через 4–6 нед после родов и исключении иной этиологии. Приблизительно в 15% случаев ВХБ выявляются генетические варианты одного из канальцевых транспортных белков: ABCB11 (белка, выводящего соли желчных кислот) или ABCB4 (фосфатидилхолинфлиппазы). У пациенток с ВХБ также обнаружены генетические варианты и/или гетерозиготность по мутациям гена белка ABCC2 (транспортера конъюгированных органических анионов), ATP8B1 (FIC1) и ядерного рецептора желчных кислот (фарнезоидного X-рецептора). Для клинической практики представляет интерес тот факт, что мутации ABCB4 обычно сопровождаются повышением уровня γ-глутамилтрансферазы (ГГТ) в сыворотке, тогда как при мутациях ABCB11, ATP8B1 и фарнезоидного X-рецептора уровень ГГТ остается низким [1].
Для дифференциальной диагностики ВХБ с вирусными инфекциями необходимо провести серологические исследования на наличие гепатитов А, В, С, вируса Эпштейна–Барра (EBV), цитомегаловируса (CMV).
Для исключения иммунологических заболеваний проводится анализ на ANA, AMA, aSMA.
Инструментальные исследования при постановке диагноза ВХБ имеют вспомогательный характер. Относительно необходимости проведения этих исследований при подозрении на ВХБ в настоящее время имеются следующие рекомендации [3]:
- ультразвуковое исследование (УЗИ) – безопасный и предпочтительный метод визуализации оценки патологических изменений печени и желчевыводящих путей при беременности. При проведении УЗИ визуализируется эхогенность и эхоструктура печени (как правило, не изменены), состояние желчных протоков печени (обычно не расширены);
- магнитно-резонансная томография (МРТ) без контрастирования может применяться во II и III триместрах беременности. Однако по диагностической ценности обычная МРТ печени без контраста приближается к УЗИ. Поэтому ее рационально рассматривать только как дополнительный метод в диагностике ВХБ, когда результаты УЗИ неоднозначны, не точны или дают недостаточно информации;
- биопсия печени выполняется в редких случаях: при тяжелом течении ВХБ или проведении дифференциальной диагностики генеза поражения печени. Морфологически выявляются центролобулярный холестаз и желчные пробки в мелких желчных канальцах, которые могут быть расширены. Гепатоцеллюлярный некроз и признаки воспаления обычно отсутствуют. После родоразрешения гистологическая картина возвращается к варианту нормы [2, 14].
ЛЕЧЕНИЕ
Цели лечения при ВХБ – купирование симптомов заболевания, улучшение или нормализация качества жизни, а также снижение риска осложнений для организма матери и плода [2].
После исключения заболеваний и состояний, таких как вирусные гепатиты, воздействие гепатотоксических лекарств (например, передозировка парацетамола), желчнокаменная болезнь, ПЭ и острая жировая дистрофия печени, предлагается следовать алгоритму лечения, показанному на рис. 4 [15].
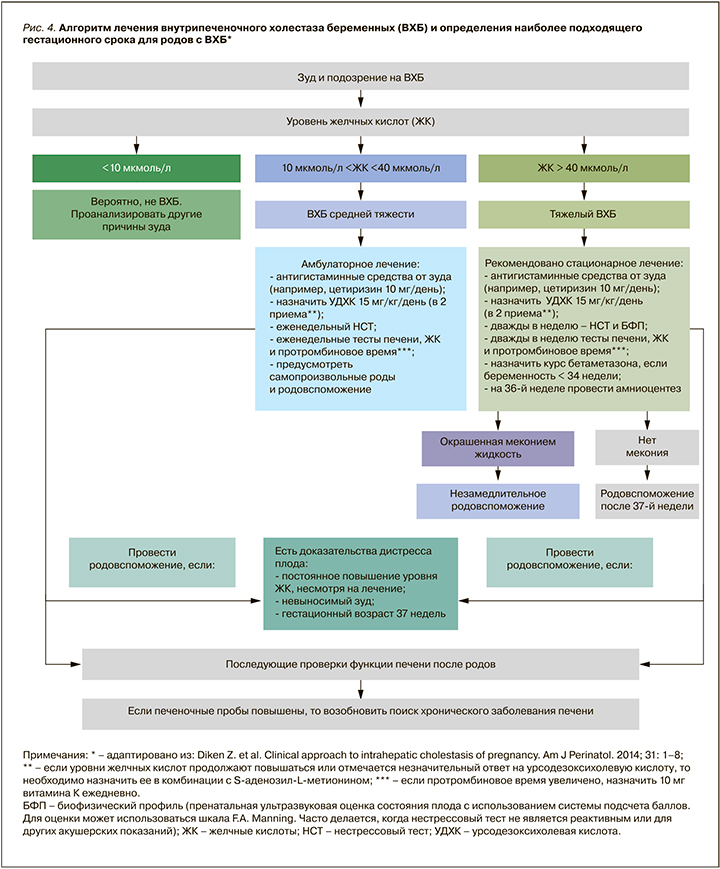
Согласно клиническим рекомендациям Российской гастроэнтерологической ассоциации (РГА) и Российского общества по изучению печени (РОПИП) по диагностике и лечению холестаза, терапия первой линии – применение урсодезоксихолевой кислоты (УДХК) в дозе 10–20 мг/кг/сут [14]. На фоне лечения УДХК наблюдается уменьшение выраженности кожного зуда, у 67–80% пациенток с ВХБ происходит снижение уровня печеночных проб; также на фоне приема этого лекарственного средства уменьшается риск неблагоприятных последствий для матери и плода [1, 2, 14–19].
Эффект УДХК обусловлен несколькими механизмами. Она усиливает транспорт желчных кислот, оказывает антиапоптозное действие и улучшает экскрецию метаболитов, вызывающих зуд, в частности сульфатов прогестерона. Данные метаанализа [18] свидетельствуют о снижении частоты преждевременных родов, аномалий сердечного ритма плода и необходимости в госпитализации новорожденных при применении УДХК и уменьшении повреждения плаценты.
Рифампицин является мощным агонистом прегнанового X-рецептора. Полагают, что сочетание этого препарата с УДХК усиливает ее благоприятное действие при необструктивном холестазе благодаря синергическому эффекту. Добавление к УДХК рифампицина ослабляет симптомы ВХБ и улучшает биохимические показатели приблизительно в 1/3 случаев, не поддающихся монотерапии УДХК [1].
Одним из препаратов УДХК, широко применяемых в терапии холестатических заболеваний печени, является Гринтерол® (АО «Гриндекс», Латвия) [20, 21]. Гринтерол® представляет собой микронизированную УДХК полного цикла европейского производства (от субстанции до готовой формы). Процесс микронизации позволяет получать однородные по размерам и чистые микрочастицы, улучшающие параметры биодоступности препарата.
Необходимо отметить, что, согласно классификации Food and Drug Administration (FDA), категория использования УДХК при беременности – B (экспериментальные исследования не выявили тератогенного действия; возможно применение препарата строго по показаниям). В соответствии с инструкцией по медицинскому применению лекарственного препарата Гринтерол® его применение у беременных возможно в случаях, когда это явно необходимо. Оптимальная доза УДХК для лечения ВХБ не определена, хотя в большинстве случаев она составляет 500 мг 2 раза/сут или 15 мг на 1 кг массы тела в день (10–20 мг/кг/сут) [2, 14]. Если зуд не удается купировать в течение нескольких дней на фоне стандартной терапии УДХК, дозу препарата можно увеличить до 25 мг/кг/сут [14].
ЗАКЛЮЧЕНИЕ
Таким образом, ВХБ – это специфическое заболевание печени при беременности. Генетические, гормональные факторы и факторы окружающей среды, по-видимому, взаимодействуют в этиопатогенезе заболевания, хотя точная его этиология все еще остается неясной. ВХБ представляется как диагноз исключения, который основывается на анализе клинических и лабораторных данных, указывающих на гепатобилиарное расстройство, возникающее при беременности. Облегчение симптомов материнского зуда, предотвращение предродовых и послеродовых геморрагических осложнений, обеспечение тщательного наблюдения течения беременности во избежание осложнений у плода (т.е. дистресса плода, внезапной внутриутробной гибели плода и преждевременных родов) служат основными принципами лечения ВХБ. УДХК является наилучшим доступным терапевтическим средством, с доказанной безопасностью и эффективностью в уменьшении зуда, восстановлении аномальных уровней желчных кислот в сыворотке крови и функциональных тестов печени. Быстрый и правильный диагноз с соответствующим медицинским вмешательством – обязательное условие для улучшения прогноза развития плода. Необходимы дальнейшие клинические испытания с высоким уровнем доказательной базы для совершенствования комплексного, научно обоснованного подхода к разработке стратегий диагностики и лечения ВХБ.



