Хроническая сердечная недостаточность (ХСН) остается серьезной проблемой современного здравоохранения из-за высокой инвалидизации и летальности. Ежегодно она становится причиной госпитализаций 1 млн пациентов, что сопряжено с высокими материальными затратами [1]. К тому же летальность в течение первого месяца после выписки из стационара достигает 10%, несмотря на существенные достижения в лечении и профилактике ХСН [2].
В России с 2001 г. под эгидой Общества специалистов по сердечной недостаточности (ОССН) проведен ряд эпидемиологических исследований, включая такие, как ЭПОХА-ХСН [3], ЭПОХА-О-ХСН [4] и ЭПОХА-Декомпенсация-ХСН [5]. Основной их целью был анализ встречаемости, особенностей течения ХСН и ассоциированных с ней клинических, социальных и психологических проблем.
За 16 лет в России распространенность ХСН выросла с 4,9% в 1998 г. до 10,2% в 2014 г. (р=0,01). За тот же период было отмечено увеличение числа пациентов с ХСН III–IV функциональных классов с 1,2 до 4,1% (р=0,002), а общая смертность больных ХСН достигла 25,1% [6].
Нарастание случаев ХСН связано с демографическим старением населения, индустриализацией общества, увеличением сердечно-сосудистых заболеваний, эффективностью современных методов лечения, развитием системы первичных сосудистых отделений и телемедицинских технологий. В совокупности эти причины приводят к увеличению продолжительности жизни и риску развития ХСН. Кроме того, в последние годы наблюдается увеличение лиц с множественной сопутствующей патологией, что предопределяет увеличение числа пациентов с более тяжелым течением ХСН и требует усовершенствования подходов к лечению [6, 7].
Цель предлагаемой статьи – обсуждение возможностей и перспектив применения комбинированного лекарственного средства сакубитрил/валсартан у пациентов с разными фенотипами ХСН.
ХРОНИЧЕСКАЯ СЕРДЕЧНАЯ НЕДОСТАТОЧНОСТЬ И МУЛЬТИМОРБИДНОСТЬ
ХСН – это мультифакторный и многокомпонентный синдром, включающий сочетание морфологических, функциональных, нейрогуморальных, клеточных и других изменений в функционировании сердца, что повышает вероятность развития других состояний и осложнений.
ХСН не развивается первично и всегда становится исходом определенного заболевания. В связи с этим вполне обосновано считать, что в большинстве случаев пациенты с ХСН являются мультиморбидными. Сердечная недостаточность провоцирует прогрессирование многих заболеваний, и в последние годы подтвержден ее вклад в глобальное «бремя» болезней (отношение шансов (ОШ) 38,4; 95% доверительный интервал (ДИ): 37,1–39,7) [8].
Крупнейшее шотландское поперечное исследование [9], которое охватило базу данных 1 424 378 пациентов в возрасте 18 лет и старше, наблюдающихся в условиях оказания первичной медицинской помощи, показало, что у 17 285 (1,2%) лиц имелась сердечная недостаточность с систолической дисфункцией левого желудочка. Группу контроля составили 1 404 471 лицо без признаков сердечной недостаточности. После поправки на возраст, пол и социальную принадлежность была установлена более частая встречаемость сопутствующей патологии у лиц с ХСН: три заболевания регистрировались у 17,5% пациентов с ХСН и только у 6,8% в группе контроля (OШ 1,22; 95% ДИ: 1,77–1,88). Наибольшая разница между группами был обнаружена для семи или более сопутствующих заболеваний (группа с ХСН – 13,9%, группа контроля – 1,1%; OШ 4,10; 95% ДИ: 3,90–4,32).
Ни одной сопутствующей патологии не было у 3,2% пациентов с ХСН и 52,0% в группе контроля (ОШ 0,90; 95% ДИ: 0,10–0,80), по одному заболеванию отмечалось у 10,6 и 21,4% соответственно (OШ 0,47; 95% ДИ: 0,45–0,50), по два – у 14,8 и 11,5% соответственно (OШ 0,77; 95% ДИ: 0,73-0,81).
Ишемическая болезнь сердца (OШ 7,98; 95% ДИ: 7,72–8,25) фибрилляция предсердий (OШ 6,84; 95% ДИ: 6,57–7,12) и хроническая болезнь почек (OШ 3,81; 95% ДИ: 3,60–4,04) оказались наиболее частыми сопутствующими патологиями у лиц с ХСН. Также у них чаще по сравнению с группой контроля наблюдалась встречались хроническая боль (OШ 3,01; 95% ДИ: 2,90–3,12) и хроническая обструктивная болезнь легких (OШ 2,65; 95% ДИ 2,38–2,51). Полученные результаты подтверждают более высокую распространенность мультиморбидности у пациентов с сердечной недостаточностью, что следует учитывать при выборе тактики лечения.
В общей европейской популяции в возрасте 60 лет и старше около 5% лиц страдают хронической сердечной недостаточностью с сохраненной фракцией выброса левого желудочка (ХСНсФВЛЖ). Этот фенотип заболевания имеется практически у каждого второго пациента с ХСН [10, 11]. Ожидается увеличение количества пациентов с ХСНсФВЛЖ в связи с демографическим старением населения и нарастанием таких социально-значимых заболеваний, как ожирение и сахарный диабет [12, 13].
ХСНсФВЛЖ рассматривается как сложный синдром с множественными факторами риска и причинами, возникающими у пациентов с мультиморбидностью. Ключевое место в ее патогенезе отводится экстракардиальным заболеваниям, которые провоцируют и поддерживают хроническое неинфекционное воспаление. Последнее, в свою очередь, вызывает системную эндотелиальную дисфункцию, нарушение коронарной микроциркуляции, развитие оксидативного стресса, фиброза миокарда и прогрессирование диастолической дисфункции ЛЖ. При этом возможно формирование любого типа ремоделирования, вопреки историческим предубеждениям о формировании только концентрической гипертрофии ЛЖ при таком фенотипе ХСН (рис. 1) [14, 15].
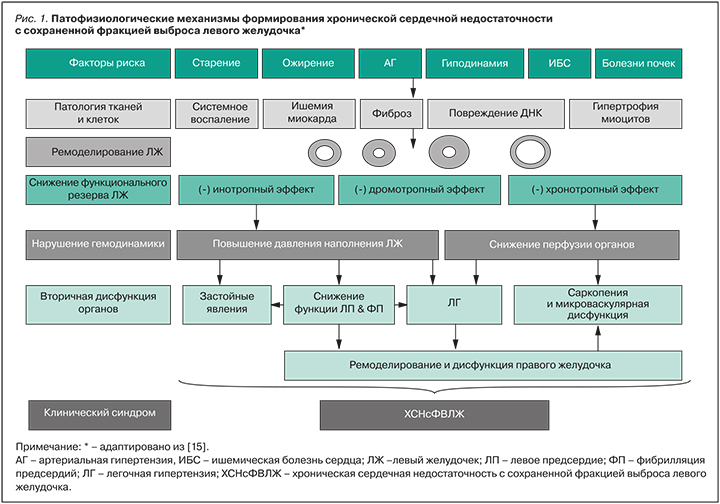
ХСНсФВЛЖ служит одной из основных причин госпитализаций, инвалидности, особенно среди лиц старшего возраста, и связана с неблагоприятным прогнозом [15, 16].
При рассмотрении мультиморбидных больных с разными фенотипами ХСН неизбежно возникает вопрос о выборе оптимальной и эффективной с точки зрения доказательной медицины медикаментозной терапии. При этом следует учитывать необходимость минимизации нежелательных явлений, риска ятрогении для обеспечения удовлетворительной переносимости лекарственных препаратов пациентом.
ИНГИБИТОР АНГИОТЕНЗИНОВЫХ РЕЦЕПТОРОВ И НЕПРИЛИЗИНА – САКУБИТРИЛ/ВАЛСАРТАН
В 2015 г. FDA (Food and Drug Administration) одобрило к применению для лечения пациентов с ХСН II–IV функционального класса (ФК) по NYHA и низкой ФВ ЛЖ комбинированное лекарственное средство, содержащее антагонист рецепторов неприлизина (нейтральной эндопептидазы) и ангиотензина II типа 1, – сакубитрил/валсартан (в англоязычной литературе этот класс лекарственных средств получил название ARNI – сокр. от Angiotensin Receptor and Neprilysin Inhibitors). Это решение было основано на результатах исследования PARADIGM-HF [17].
В 2016 г. в гайдлайнах Европейского общества кардиологов (ESC) по диагностике и лечению острой и хронической сердечной недостаточности сакубитрил/валсартан был отнесен к группе основных препаратов, доказавших способность снижать смертность и заболеваемость при ХСН с ФВ ЛЖ <40%, и рекомендован с целью снижения риска госпитализаций в связи с декомпенсацией ХСН и летального исхода (класс I, уровень B) [18]. В настоящее время он одобрен к применению более чем в 57 странах мира.
Действие препарата опосредуется одновременным подавлением активности неприлизина веществом LBQ657 (активный метаболит – сакубитрил) и блокадой рецепторов ангиотензина II типа 1 валсартаном (рис. 2).
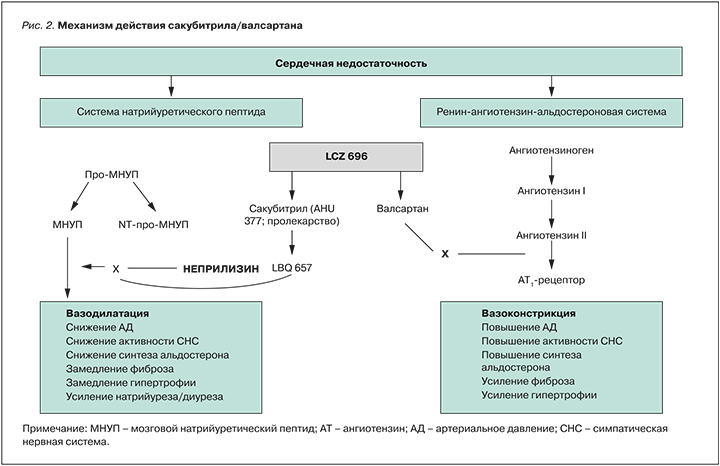
После приема внутрь сакубитрил/валсартан распадается на сакубитрил, сакубитрилат и валсартан, которые достигают пика концентрации в плазме крови через 30 мин, 2 и 1,5 ч соответственно. Абсолютная биодоступность сакубитрила составляет около 60%. При использовании препарата 2 раза/сут стабильная концентрация в крови сакубитрила, сакубитрилата и валсартана достгигается через 3 дня. Прием пищи не оказывает клинически значимого действия на компоненты сакубитрила/валсартана, которые тесно связываются с белками плазмы (94–97%).
После попадания в организм от 52 до 68% сакубитрила (преимущественно сакубитрилата) и около 13% валсартана и его метаболитов экскретируются с мочой; от 37 до 48% сакубитрила (преимущественно сакубитрилата) и около 86% валсартана выводятся через кишечник. Период полувыведения сакубитрила, сакубитрилата и валсартана составляет 1,4, 11,5 и 9,9 ч соответственно [19].
Сакубитрил/валсартан вызывает огромный интерес у многих исследователей, и в настоящее время проводится целый ряд испытаний по изучению его безопасности и эффективности у пациентов с ХСН и разной ФВ ЛЖ.
ВОЗМОЖНОСТИ ПРИМЕНЕНИЯ САКУБИТРИЛА/ВАЛСАРТАНА ПРИ ХРОНИЧЕСКОЙ СЕРДЕЧНОЙ НЕДОСТАТОЧНОСТИ С НИЗКОЙ ФРАКЦИЕЙ ВЫБРОСА ЛЕВОГО ЖЕЛУДОЧКА
Как сказано выше, данные о высокой эффективности и безопасности сакубитрила/валсартана были получены в международном многоцентровом двойном слепом плацебо-контролируемом клиническом исследовании с участием 8442 пациентов с ХСН – PARADIGM-HF [17]. В нем приняли участие пациенты в возрасте 18 лет и старше с ХСН II– IV ФК по NYHA, ФВ ЛЖ ≤40%, уровнем натрийуретического пептида (НУП) не менее 150 пкг/мл или N-терминального про-НУП (NT-proBNP) не менее 600 пкг/мл; при этом в случае госпитализации в связи с сердечной недостаточностью в течение предшествующего года, уровень НУП должен был быть не менее 100 пкг/ мл, а NT-proBNP – не менее 400 пкг/мл. Пациенты принимали ингибиторы ангиотензинпревращающего фермента (ИАПФ) или антагонисты рецепторов ангиотензина II (АРА), причем в течение не менее 4 нед до первого обследования должны были находиться на стабильной терапии бета-адреноблокаторами (ББ) и ИАПФ (или АРА II) в дозе, эквивалентной не менее 10 мг эналаприла. Результаты исследования подтвердили более высокую эффективность сакубитрила/валсартана в сравнении с эналаприлом. Так, в основной группе пациентов, принимавших исследуемый препарат, смерть от сердечно-сосудистых причин или госпитализация в связи с ХСН были зафиксированы в 914 (21,8%) случаях, тогда как в группе эналаприла – в 1117 (26,5%) (ОШ 0,80; 95% ДИ: 0,73–0,87; р <0,001).
В группе приема сакубитрила/валсартана госпитализация в связи с сердечной недостаточностью потребовалась 537 пациентам (12,8%), эналаприла – 658 (15,6%) (ОШ 0,79; 95% ДИ; 0,71–0,89; p <0,001). За период наблюдения (медиана 27 мес) в группе сакубитрила/валсартана умерло 558 больных (13,3%), эналаприла – 693 (16,5%) (ОШ 0,80; 95% ДИ: 0,71–0,89; р <0,001).
Авторами было сделано заключение, что для предупреждения одной госпитализации или одной смерти от сердечно-сосудистых причин на протяжении периода наблюдения прием сакубитрила/валсартана потребовался бы 21, а эналаприла – 32 пациентам.
Результаты исследования PARADIGM-HF позволили рассматривать сакубитрил/валсартан в качестве надежной альтернативы ИАПФ при наличии клинических симптомов ХСН и ФВ ЛЖ ≤35% у амбулаторных пациентов, которые принимают в оптимальной дозировке ИАПФ/АРА II в сочетании с ББ и антагонистами минералокортикоидных рецепторов (АМКР).
Спустя год после публикации первых результатов исследования PARADIGM-HF Packer M. et al. подтвердили преимущество приема сакубитрила/валсартана над ИАПФ у пациентов с ХСН и низкой ФВ ЛЖ. В группе сакубитрила/валсартана по сравнению с группой эналаприла число госпитализаций в связи с декомпенсацией ХСН было ниже на 23% (851 против 1079; p <0,001), необходимость в оказании интенсивной терапии – на 18% (768 против 879, р=0,005), парентерального введения инотропных препаратов – на 31% (р <0,001), имплантации вспомогательных устройств или пересадки сердца – на 22% (р=0,07) [20].
На сегодня практически все мультимаркерные диагностические и прогностические подходы к сердечной недостаточности включают оценку уровня НУП, отражающего изменения нейрогуморальной активности. Неприлизин рассматривается как альтернатива НУП и показано, что высокая концентрация его растворимой формы ассоциирована с сердечно-сосудистой заболеваемостью и летальностью при сердечной недостаточности.
В исследовании Bayes-Genis A. et al. с участием 797 амбулаторных пациентов с ХСН уровень неприлизина (медиана 0,64 (0,42–1,23) нг/ мл) положительно коррелировал с возрастом (p <0,001) и ST2 (p <0,001). При многофакторной регрессии Кокса, в которую вошли клинические параметры, высокочувствительный тропонин, ST2 и растворимый неприлизин, на протяжении 4,7 лет наблюдения пациентов сохранялась независимая ассоциация концентрации неприлизина с комбинированной конечной точкой – сердечно-сосудистой смертностью и госпитализациями по поводу сердечной недостаточности (ОШ 1,14; 95% ДИ: 1,02–1,27; p=0,03), а также сердечно-сосудистой смертностью (ОШ 1,15; 95% ДИ: 1,01–1,31; p=0,04). Характерно, что для NT-proBNP такой ассоциации получено не было. Полученные результаты позволили авторам подтвердить значимость неприлизина в качестве независимого предиктора в мультимаркерной стратегии при определении прогноза у лиц с ХСН [21].
Неприлизин (нейтральная эндопептидаза; энкефалиназа; энкефалиназа A) представляет собой цинк-зависимую металлопептидазу, синтезируемую эндотелием сосудов. Он катализирует деградацию ряда пептидов с вазодилатирующими свойствами: НУП, ангиотензина I и II, брадикинина, субстанции Р, адреномедуллина, вазоактивного интестинального полипептида и эндотелина-1; это приводит к активации нейрогуморальных систем, вазоконстрикции, задержке натрия и воды, патологическому ремоделированию органов кровообращения.
В свою очередь, эффекты НУП включают снижение уровня АД через воздействие на тонус сосудистого русла и водно-электролитный баланс, антипролиферативный и антифибротический эффекты. Развитие ряда сердечно-сосудистых заболеваний ассоциировано с нарушением регуляции системы НУП, в связи с чем для достижения ее дополнительных кардио- и нефропротективных эффектов возможно применение ингибиторов неприлизина. Угнетение неприлизина приводит к усилению натрийуретического, диуретического и вазодилатирующего эффектов эндогенного НУП и в результате к снижению АД [22].
Как уже отмечалось, что неприлизин принимает участие в деградации и других вазоактивных пептидов, в частности ангиотензина I, II и эндотелина-1. Поэтому баланс эффектов воздействия на сосудистый тонус ингибиторов неприлизина достаточно вариабельный и зависит от превалирования констрикторных и дилатирующих влияний. В связи с этим сочетание эффектов ИАПФ/АРА и ингибиторов неприлизина может существенно потенцировать гемодинамические и антипролиферативные эффекты в результате комплементарного механизма действия [23].
Результаты исследования PIONEER-HF показали, что у пациентов (n=440) с остро декомпенсированной ХСН и низкой ФВ ЛЖ терапия ингибитором ангиотензиновых рецепторов и неприлизина (АРНИ) по сравнению с эналаприлом способствовала более выраженному снижению концентрации NT-proBNP и при этом не ассоциировалась с риском появления нежелательных явлений в виде нарушения функции почек или артериальной гипотензии [24].
Последующий анализ подтвердил, что начало приема сакубитрила/валсартана в госпитальных условиях служит залогом применения более низкой дозы этого препарата в будущем. Как показали результаты, сакубитрил/валсартан в более низкой дозе (50 мг/12 ч) чаще принимали госпитализированные пациенты, нежели амбулаторные (80,8 против 48,8%; р <0,001). Было сделано заключение, что если пациент начинает прием сакубитрила/валсартана в амбулаторных условиях, то этот факт возможно рассматривать в качестве предиктора применения более высокой дозы в будущем – 100 или 200 мг 2 раза/сут (OШ 3,1; 95% ДИ: 1,7–5,6; р <0,001) [25].
Несмотря на то что АРНИ показали благоприятные эффекты в плане снижения смертности у пациентов с ХСН и низкой ФВ ЛЖ, данных, подтверждающих аналогичный эффект у пациентов после перенесенного инфаркта миокарда разной давности или с подтвержденной ишемической болезнью сердца в настоящее время, недостаточно. Однако следует отметить, что среди пациентов, включенных в две сравнимаемые группы исследования PARADIGM-HF, около 60% имели ХСН с низкой ФВ ЛЖ ишемического генеза.
В настоящее время ожидаются результаты крупнейшего многоцентрового (41 страна), двойного слепого, активно контролируемого проспективного рандомизированного клинического исследования PARADISE‐MI с участием пациентов, перенесших инфаркт миокарда, у которых развилась систолическая дисфункция ЛЖ (ФВ ЛЖ ≤40%). В нем 5669 пациентов в возрасте 64±12 лет (24% – женщины) с ФВ ЛЖ 37±9% рандомизированы в группу приема сакубитрила/валсартана или рамиприла. Цель этого исследования – определить, действительно ли сакубитрил/валсартан превосходит по эффективности рамиприл в отношении предупреждения развития сердечно-сосудистой смертности, госпитализаций по поводу ХСН или декомпенсации ХСН у амбулаторных пациентов после перенесенного инфаркта миокарда [26].
Выделение группы пациентов, чувствительных к эффектам сакубитрила/валсартана, является весьма актуальной проблемой, а разработка шкалы, предсказывающей высокий ответ пациентов на прием этого препарата, облегчит выбор рациональной терапии практикующим врачам.
Согласно данным исследования, Moliner-Abоs C. et al. [27] с участием 185 амбулаторных больных с ХСН II–IV ФК по NYHA и ФВ ЛЖ ≤40%, высокочувствительными к эффектам сакубитрила/валсартана оказались 65 (35,1%) пациентов, которые были сопоставимы по возрасту с пациентами, имеющими стандартный ответ на терапию этим препаратом (65±13 против 67±12 лет; р=0,354). Среди них статистически незначимо чаще были женщины (31 и 22% соответственно; р=0,171). На фоне лечения у 29 пациентов, высокочувствительных к эффектам сакубитрила/валсартана, уровень NT-proBNP снизился на 50% и более от исходного, у 26 улучшилась ФВ ЛЖ, у 10 наблюдалось одновременное снижение указанного биомаркера и улучшение ФВ ЛЖ.
Многофакторный логистический регрессионный анализ позволил выявить независимые предикторы высокочувствительного ответа на прием сакубитрила/валсартана: это отсутствие в схеме лечения АМКР (OШ 2,3; 95% ДИ: 1,1–4,8) и I–II ФК по NYHA (OШ 2,3; 95% ДИ: 1,1–4,8; p=0,025). Помимо этих двух факторов, модель включала женский пол (β-коэффициент 0,536; ОШ 1,7; 95% ДИ: 0,8–3,6; p=0,161), госпитализации по поводу декомпенсации ХСН в течение 2 предшествующих лет (β-коэффициент 0,601; ОШ 1,8; 95% ДИ: 0,9–3,6; p=0,088), синусовый ритм по ЭКГ (β-коэффициент 0,692; ОШ 2,0; 95% ДИ: 1,0–4,0; p=0,052) и отсутствие приема диуретиков (β-коэффициент 0,743; ОШ 2,1; 95% ДИ: 1,0–4,5; p=0,059). Результаты данного исследования легли в основу шкалы The AWARD-class score, позволяющей оценить шанс наличия высокой чувствительности к сакубитрилу/валсартану среди пациентов с ХСН и низкой ФВ ЛЖ с учетом простых показателей, широко применяемых в клинической практике: пола (5 баллов – женский пол), ФК ХСН (8 баллов), синусового ритма сердца (7 баллов), госпитализаций в анамнезе (6 баллов), отсутствия приема АМКР (8 баллов) и диуретиков (7 баллов).
Таким образом, не вызывает сомнений достаточно высокая эффективность и безопасность сакубитрила/валсартана. Этот препарат рекомендован к приему в стартовой дозировке 24/26 мг 2 раза/ сут с дальнейшей ее титрацией каждые 2–4 нед до максимально переносимой (97/103 мг 2 раза/сут) у пациентов с сохраняющейся клинически выраженной ХСН и низкой ФВ ЛЖ вместо ИАПФ/ АРА, несмотря на оптимально подобранную терапию комбинацией ИАПФ/АРА, ББ и АМКР с целью снижения риска госпитализаций в связи с ХСН и летальности [28].
Преимуществом сакубитрила/валсартана является минимальная зависимость от метаболизма, опосредуемого CYP450. Это позволяет сочетать его с такими лекарственными средствами, как фуросемид, варфарин, дигоксин, карведилол, амлодипин, омепразол, гидрохлоротиазид, метформин, аторвастатин, которые необходимы в разных клинических ситуациях у пациентов с ХСН и сопутствующей патологией.
Сочетание сакубитрила/валсартана с ИАПФ противопоказано из-за риска развития ангионевротического отека. Совместное назначение калийсберегающих диуретиков (спиронолактона, триамтерена, амилорида), добавок калия или содержащей его солиможет приводить к повышению концентрации сывороточного K+. У пациентов старшего возраста, лиц, которые не принимают диуретики, или больных с хронической болезнью почек следует помнить об ухудшении функции почек при назначении нестероидных противовоспалительных средств, включая селективные ингибиторы циклооксигеназы-2.
Клинически значимые нежелательные эффекты сакубитрила/валсартана встречаются достаточно редко и включают гипотензию (18%), гиперкалиемию (12%), кашель (9%), головокружение (6%), ортостатические реакции (2,1%), ангионевротический отек (<1%). Это, безусловно, также можно отнести к достоинствам этого лекарственного средства, в том числе при лечении пациентов с множественной сопутствующей патологией [18].
ВОЗМОЖНОСТИ ПРИМЕНЕНИЯ САКУБИТРИЛА/ВАЛСАРТАНА ПРИ ХРОНИЧЕСКОЙ СЕРДЕЧНОЙ НЕДОСТАТОЧНОСТИ С СОХРАНЕННОЙ ФРАКЦИЕЙ ВЫБРОСА ЛЕВОГО ЖЕЛУДОЧКА
В настоящее время нет убедительных данных о высокой прогностической эффективности каких-либо классов лекарственных препаратов, применяемых при ХСН с сохраненной ФВ ЛЖ (ХСНсФВЛЖ), в отличие от ХСН с низкой ФВ ЛЖ. В связи с этим изучение возможностей применения новых видов терапии различных фенотипов заболевания остается одной из первостепенных целей современной медицины (табл.) [29].
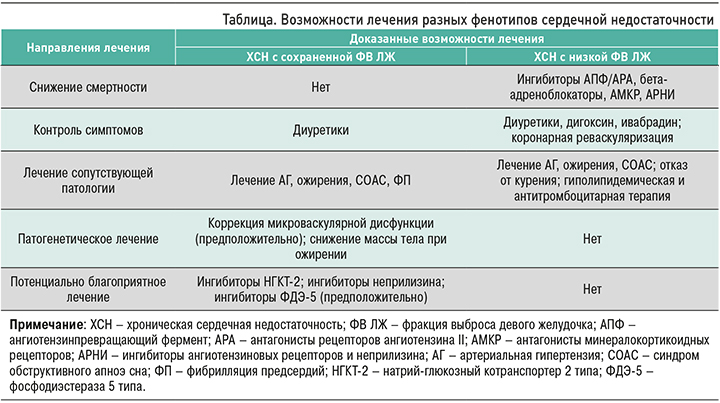
Рандомизированное двойное слепое многоцентровое исследование 2 фазы в параллельных группах PARAMOUNT с участием пациентов c ХСН II–III ФК по NYHA, ФВ ЛЖ ≥45%, NT-proBNP >400 пг / мл оценивало эффекты саубитрила/валсартана (с титрованием дозы до 200 мг 2 раза/ сут) или валсартана (с титрованием дозы до 160 мг 2 раза/сут) в отношении уровня NT-proBNP на протяжении 36 нед. К 12-й неделе лечения в группе приема сакубитрила/валсартана было отмечено статистически значимое снижение сывороточной концентрации NT-proBNP по сравнению с группой валсартана. В первом случае этот показатель с исходного уровня 783 пг/мл (95% ДИ: 670–914) уменьшился к 12-й неделе до 605 пг/мл (95% ДИ: 512–714); во втором случае аналогичные показатели составили 862 (95% ДИ: 733–1012) и 835 (95% ДИ: 710–981) пг/мл соответственно (отношение сакубитрила/валсартана к валсартану 0,77; 95% ДИ: 0,64–0,92; p=0,005). При этом было установлена сопоставимая переносимость сакубитрила/валсартана и валсартана [30]. Данные результаты легли в основу разработки будущих исследований по изучению эффектов сакубитрила/валсартана у больных ХСН и сохраненной ФВ Ж.
В мультицентровом двойном слепом сравнительном исследовании PARGON-HF сравнивались эффекты сакубитрила/валсартана (97 мг + 103 мг 2 раза/сут) и валсартана (160 мг 2 раза/ сут) у 4822 пациентов с ХСН II–IV ФК по NYHA и ФВ ЛЖ ≥45%. В качестве конечных событий рассматривались госпитализации в связи с декомпенсацией ХСН и сердечно-сосудистая смертность. Результаты не показали преимуществ того или иного варианта лечения (ОШ 0,87; 95% ДИ: 0,75–1,01; p=0,06); в то же время было отмечено, что у пациентов, получавших сакубитрил/валсартан, чаще встречались гипотензия и ангионевротический отек, но реже гиперкалиемия [31]. Возможно, что одно из существенных ограничений данного исследования, а именно невключение в критерии исключения таких специфических причин ХСН с сохраненной ФВ ЛЖ и диастолической дисфункции, как амилоидоз и болезнь Фабри, могло оказать существенное влияние на полученные результаты из-за резистентности пациентов к сакубитрилу/валсартану.
В январе 2021 г. опубликованы данные ретроспективного анализа пациентов, получавших или не получавших АМКР дополнительно к сакубитрилу/валсартану, который сравнивался с валсартаном в рамках исследования PARAGON-HF [32]. Исходно АМКР применяли 1239 (26%) больных ХСН, не использовали 3557 (74%). Пациенты в группе приема АМКР были моложе, чем в группе неприема АМКР (72 года против 73 лет; p <0,001), среди них чаще встречались лица мужского пола (52 против 47%; p <0,001), они имели более низкую ФВ ЛЖ (57 против 58%; p <0,001) и более высокую частоту госпитализаций по поводу сердечной недостаточности (59 против 44%; p <0,001). В исследовании была показана эффективность сакубитрила/валсартана в комбинации с АМКР в плане предупреждения ежегодного снижения расчетной скорости клубочковой фильтрации (абсолютная разница в пользу сакубитрила/валсартана +1,2 мл/мин/1,73 м2 в год; 95% ДИ: 0,6–1,7).
Кроме того, нефропротективный эффект сакубитрила/валсартана был продемонстрирован в ретроспективном когортном исследовании с участием 274 пациентов (65,7% мужчины; средний возраст 72,7 лет) с ХСН. Средняя скорость клубочковой фильтрации (СКФ) в группе приема сакубитрила/валсартана составила 70,9±24,6 мл/ мин/1,73 м2, валсартана – 71,0±24,9 мл/мин/1,73 м2 (p=0,97). ФВ ЛЖ в группе приема сакубитрила/валсартана составила 52,7±15,7%, валсартана – 55,4±15,6 (р=0,15). Период наблюдения составил 18 мес. В группе приема сакубитрила/валсартана, по сравнению с приемом только валсартана, наблюдалось статистически значимо более высокое значение СКФ (р <0,01). Авторы отметили очень важный факт: уровень СКФ был выше именно у пациентов с ФВ ЛЖ ≥40% или СКФ ≥60 мл/мин/1,73 м2. Многофакторный регрессионный анализ показал более низкий риск развития нарушения функции почек при приеме сакубитрила/валсартана по сравнению с приемом только валсартана (ОШ 0,5; 95% ДИ: 0,3–0,9), что позволяет рассматривать пациентов с ФВ ЛЖ ≥40% и СКФ≥60 мл/ мин/1,73 м2 как приоритетную категорию для применения сакубитрила/валсартана [33]. Таким образом, появляется дополнительная ниша для назначения саакубитрила/валсартана пациентам с сохраненной и промежуточной ФВ ЛЖ и ХБП 1–2 стадии, ассоциированной со многими сердечно-сосудистыми и экстракардиальными патологиями, что позволит замедлить прогрессирование заболевания.
Как сказано выше, блокаторы неприлизина способны предотвращать распад некоторых вазоактивных нейропептидаз, которые обладают противовоспалительной активностью и усиливают липолитический эффект. Также действие ингибиторов неприлизина реализуется через снижение уровня альдостерона и блокады его действия. Теоретически эти благоприятные эффекты уменьшают объем плазмы и улучшают микрососудистую функцию у пациентов с сохраненной ФВ ЛЖ [29].
Международное многоцентровое рандомизированное двойное слепое контролируемое исследование PARALLAX, стартовавшее в августе 2017 г., сравнивало эффекты сакубитрила/валсартана с комплексной индивидуализированной медикаментозной терапией, включающей эналаприл или валсартан или плацебо, у 2572 больных ХСН с промежуточной/сохраненной ФВ ЛЖ (более 40%, в среднем 56%) и сопутствующей патологией в возрасте 45 лет и старше (средний возраст 73 года, 51% – женщины). Целью исследования была оценка влияния сакубитрила/валсартана на снижение NT-proBNP, улучшение переносимости физической нагрузки и облегчение симптомов ХСН с дальнейшим повышением качества жизни на протяжении 24 нед наблюдения. В исследовании было достигнуто одно из двух запланированных конечных событий: через 12 нед терапии сакубитрилом/валсартаном уровень NT-proBNP снизился на 16,4% (ОШ 0,84; 95% ДИ: 0,80–0,88; p <0,0001). При этом через 24 нед в сравниваемых группах наблюдалось сопоставимое увеличение дистанции с 6-минутной ходьбой: скорректированная средняя разница составила 2,5 м: 9,7 м в группе сакубитрила/валсартана и 12,2 м в группе сравнения (р=0,42) [34]. Несмотря на неоправданные ожидания от этого исследования, результаты post-hoc анализа показали, что риск декомпенсации ХСН с последующей госпитализацией был ниже в группе саакубитрила/валсартана (ОШ 0,49; 95% ДИ: 0,30–0,81). Возможно, что будущие исследования позволят выделить группу пациентов, у которых применение сакубитрила/валсартана будет эффективным. Пока в эту категорию входят женщины, пациенты, которые по каким-либо причинам резистентны к приему диуретиков, и лица с частыми госпитализациями [35, 36].
Объединенный анализ результатов двух крупнейших исследований, описанных выше PARADIGM-HF (критерий включения – ФВ ЛЖ ≤40%; n=8399) и PARAGON-HF (критерий включения – ФВ ЛЖ ≥45%; n=4796), подтвердил более низкую частоту госпитализаций в связи с декомпенсацией ХСН (ОШ 0,84; 95% ДИ: 0,78–0,90), сердечно-сосудистой смертности (ОШ 0,84; 95% ДИ: 0,76–0,92) и общей смертности (ОШ 0,88; 95% ДИ: 0,81–0,96) при приеме сакубитрила/валсартана у более чем 15 тыс. пациентов, большинство которых имели АГ, каждый третий – фибрилляцию предсердий, сахарный диабет и каждый десятый – инсульт в анамнезе. Благоприятные эффекты сакубитрила/валсартана чаще регистрировались у женщин по сравнению с мужчинами, что также позволяет предположить его более высокую эффективность у пациентов женского пола [37].
Причины гендерных особенностей в эффективности сакубитрила/валсартана остаются неопределенными. Поскольку у женщин нормальные значения ФВ ЛЖ обычно выше, чем у мужчин, возможно, что у первых систолическая дисфункция ЛЖ может развиваться при более высоком значении ФВ ЛЖ, чем у вторых. Этот аспект требует дальнейшего изучения и подтверждения.
ЗАКЛЮЧЕНИЕ
АРНИ представляют собой современный класс лекарственных средств с высокой безопасностью и эффективностью у больных ХСН. Одновременная блокада рецепторов ангиотензина II и неприлизина оказывает благоприятные воздействия на прогноз и снижение частоты госпитализаций у пациентов в возрасте 18 лет и старше с ХСН, сопутствующей патологией и разными показателями ФВ ЛЖ. Эти эффекты могут быть связаны как с действием сакубитрила/валсартана на показатели гемодинамики, так и изменением функции почек и ряда метаболических параметров.
Актуальнейшая задачая на сегодняшний день – выделение высокочувствительной к эффектам сакубитрила/валсартана группы пациентов с ХСН. Существующие данные свидетельствуют о том, что лица женского пола, пациенты с недлительным анамнезом сердечной недостаточности и I–II ФК ХСН могут рассматриваться в качестве целевой группы, у которой применение сакубитрила/валсартана сопряжено с улучшением прогноза и снижением частоты госпитализаций. Крупнейшая «зонтичная» клиническая программа FortiHFy, в рамках которой с 2016 г. изучаются клинические особенности и качество жизни пациентов с ХСН, позволит в ближайшей перспективе уточнить преимущества сакубитрила/валсартана и открыть новые горизонты для его дальнейшего изучения.



