ВВЕДЕНИЕ
Бронхиальная астма (БА) – одно из наиболее распространенных респираторных заболеваний, которым в разных странах мира страдают около 360 млн человек. Она существенно снижает качество жизни пациентов и членов их семей, отличается высокой стоимостью лечения и ежегодно становится причиной смерти примерно 400 000 больных в мире [1]. Современное ведение пациентов с БА основано на принципах, изложенных в глобальной инициативе по БА (GINA) и российских клинических рекомендациях [2, 3].
В настоящей статье обсуждается лечение БА стабильного течения и перспективы его дальнейшего совершенствования.
ТЯЖЕСТЬ И КОНТРОЛЬ БРОНХИАЛЬНОЙ АСТМЫ
Терапия БА в настоящее время зависит от степени ее тяжести, контроля и фенотипа.
Тяжесть астмы ранее определялась на основании выраженности клинической картины и значений показателей функции легких до начала терапии. Недостаток такого подхода заключался в том, что он не учитывал особенности течения заболевания у пациентов, которые ранее уже получали лечение.
В современных международных и национальных рекомендациях тяжесть течения БА предлагается оценивать ретроспективно в течение не менее 2–3 мес с учетом объема проводимой терапии, необходимого для достижения контроля заболевания (табл. 1).
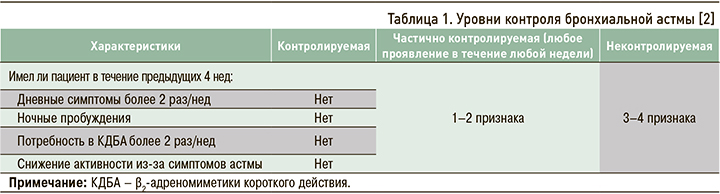
Выделяют три степени тяжести течения БА [2].
1. Легкая астма: контроль достигается при использовании комбинации ингаляционный глюкокортикостероид (ИГКС) + формотерол в режиме «по требованию» или при регулярном лечении низкими дозами ИГКС или антилейкотриеновых препаратов (АЛТ). Эксперты GINA не рекомендуют более выделять интермиттирующую и персистирующую легкую БА в связи с тем, что в обоих случаях у пациентов могут развиваться тяжелые обострения, для предупреждения которых требуется лечение ИГКС [2].
2. Астма средней тяжести: достижение контроля возможно при постоянном использовании низких и средних доз ИГКС в комбинации с β2-адреномиметиками длительного действия (ДДБА).
3. Тяжелая астма: форма заболевания, при котором контроль возможен в случае назначения высоких доз ИГКС + ДДБА или же которое остается неконтролируемым, несмотря на лечение ими [2, 4].
Основные цели терапии БА включают достижение и поддержание контроля симптомов болезни в течение длительного времени, профилактику обострений, улучшение функции легких.
Контроль БА – это выраженность клинических проявлений заболевания или степень их уменьшения/исчезновения под влиянием лечения [2]. Оценка контроля осуществляется путем анализа двух компонентов – тяжести симптомов и определения риска будущих обострений. Последний зависит от многих факторов, в том числе дисциплины пациента, правильной техники ингаляций, сопутствующих заболеваний, степени тяжести бронхиальной обструкции.
Плохой контроль БА увеличивает вероятность ее последующих обострений. Вместе с тем отсутствие симптомов у пациентов с легкой БА не исключает вероятности развития тяжелых обострений этого заболевания при воздействии триггеров (например, при контакте с аллергенами или вирусными инфекциями).
Установлено, что достижение контроля возможно при адекватной терапии у большинства пациентов. Для его оценки в реальной клинической практике рекомендуется использовать специальные вопросники – Asthma Control Test (АСТ) и Asthma Control Question-naire (ACQ-5). Они позволяют быстро и точно определять состояние больных врачами первичного звена здравоохранения и специалистами (пульмонологами и аллергологами-иммунологами).
К факторам риска обострений БА относятся высокая потребность в бронхолитиках короткого действия (более 3 ингаляторов КДБА в год), неправильное или недостаточное использование ИГКС, низкие показатели функции легких (ОФВ1 <60%), высокая степень бронхиальной гиперреактивности, значимые психологические и социально-экономические проблемы, воздействие поллютантов и этиологически значимых аллергенов, курение, серьезные сопутствующие заболевания, эозинофилия крови и/или мокроты, высокий уровень оксида азота в выдыхаемом воздухе, беременность, интенсивная терапия по поводу обострения астмы в анамнезе, не менее одного тяжелого обострения астмы за последние 12 мес [2].
Хорошо известно, что астма является гетерогенным заболеванием. Выделение особых групп пациентов по клиническим и патофизиологическим принципам лежит в основе концепции фенотипов заболевания. В нашей стране это нашло отражение в классификациях БА, предложенных А.Д. Адо, П.К. Булатовым и Г.Б. Федосеевым [5].
В международных согласительных документах выделяют следующие фенотипы БА [2]:
1) аллергическую;
2) неаллергическая;
3) позднюю;
4) астму с фиксированной бронхиальной обструкцией;
5) астму с ожирением.
Фенотипирование пациентов имеет большое значение при БА различной тяжести течения. Фенотипы болезни, наряду с тяжестью ее течения и контролем, должны указываться в диагнозе у пациентов в реальной клинической практике.
ВЕДЕНИЕ БОЛЬНЫХ АСТМОЙ
Целью терапии БА является достижение контроля и снижение риска летального исхода, обострений, формирования фиксированной бронхиальной обструкции и побочного действия лекарственных препаратов. Ведение пациентов должно быть персонализированным и строиться на непрерывном цикле оценки и мониторирования их состояния, сопутствующих заболеваний, техники ингаляции и чувствительности к лечению. Оно включает установление партнерских отношений между больным и врачом, обучение, выявление и устранение факторов риска развития обострений, оценку и достижение контроля астмы, лечение обострений, терапию особых случаев астмы (беременность, аспириновая астма и др.) и сопутствующих заболеваний. Обучение – важнейшее условие для установления партнерских отношений между врачом и пациентом, повышающее приверженность к лечению.
Устранение факторов риска развития обострений (аллергенов, инфекции, «виновных» лекарственных средств, поллютантов и др.) и лечение сопутствующих болезней (аллергического ринита, полипоз носа, аллергического бронхолегочного аспергиллеза, сердечно-сосудистых заболеваний, гастроэзофагального рефлюкс и др.) позволяет улучшить контроль болезни и сократить потребность в используемых препаратах. Меры элиминационной терапии подробно изложены в международных и национальных руководствах [2, 3].
ОБЩИЕ ПОДХОДЫ К ФАРМАКОТЕРАПИИ БРОНХИАЛЬНОЙ АСТМЫ
Основным принципом использования лекарственных средств при БА является ступенчатый подход с увеличением и снижением объема терапии в зависимости от контроля заболевания и наличия/отсутствия факторов риска обострений (рис.).
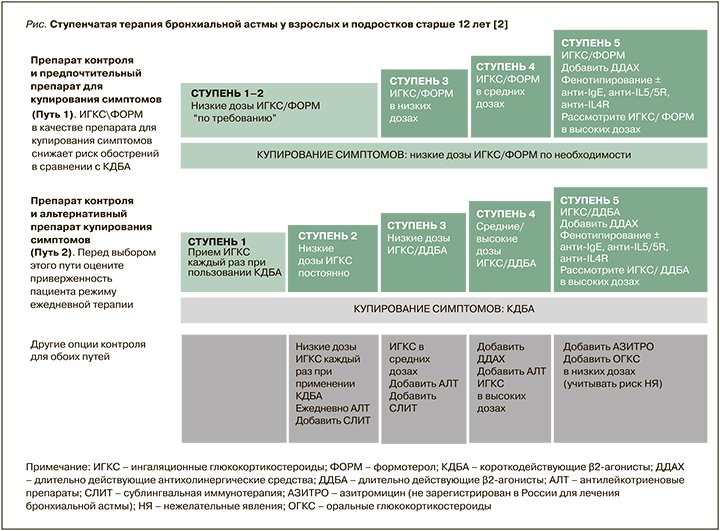
Экспертами GINA в 2021 г. предложены 2 режима лечения [2]. Первый включает применение комбинации ИГКС/формотерол для купирования симптомов на всех ступенях лечения БА. Пациенты со средней и тяжелой астмой (3–5 ступени) также используют эту комбинацию для поддерживающей терапии (режим «единого ингалятора»). Этот режим признан предпочтительным, так как по сравнению с ситуационным применением КДБА позволяет сократить число обострений болезни.
Второй режим предполагает применение для купирования симптомов КДБА. У пациентов с легкой БА (ступень 1) ингаляции КДБА должны сопровождаться использованием ИГКС. На 2–5 ступенях терапии требуется регулярное применение различных доз ИГКС в виде монотерапии (ступень 2) или в комбинации с ДДБА, АЛТ и длительно действующими холинолитиками (ступени 3–5). Использование такого режима возможно при высокой дисциплине пациента и отсутствии у него обострений астмы.
Оба рекомендованных варианта подразумевают отказ от использования монотерапии КДБА на всех ступенях лечения.
При контролируемой астме пациенту подбирают минимально необходимый уровень лечения. При частично контролируемой и неконтролируемой БА назначается более высокая ступень терапии.
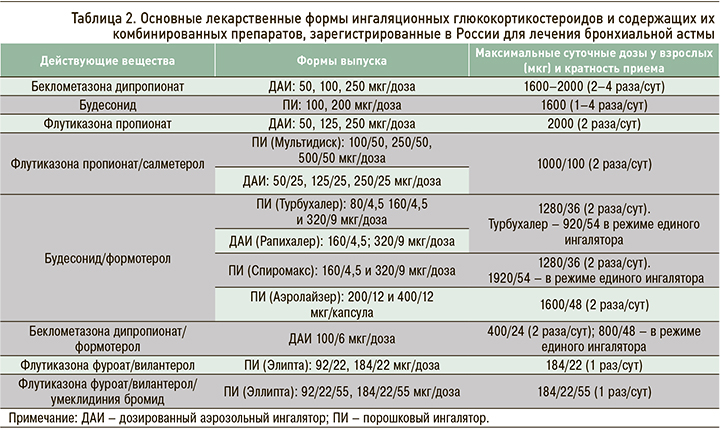
Наиболее эффективными противовоспалительными препаратами для лечения астмы любой степени тяжести, в том числе легкой, являются ИГКС. В настоящее время в нашей стране из средств этого класса используются беклометазона дипропионат, будесонид, флутиказона пропионат (табл. 2). Они уменьшают выраженность всех симптомов заболевания (частоту приступов удушья, потребность в КДБА и др.), улучшают функцию легких и снижают гиперреактивность бронхов к аллергенам и неспецифическим раздражителям (физической нагрузке, холодному воздуху и др.). Также ИГКС предупреждают развитие фиксированной бронхиальной обструкции, снижают частоту обострений астмы и госпитализаций, летальность от БА, повышают качество жизни пациентов.
Известно, что эффективность лечения ИГКС тем выше, чем раньше они назначены. Большая часть терапевтических эффектов этих препаратов развивается при их использовании в низких и средних дозах.
К сожалению, приверженность к монотерапии ИГКС в реальной клинической практике остается низкой и не превышает 25%. К основным причинам отказа пациентов от регулярного применения ИГКС относятся возможные побочные эффекты [6] и тревога за свое здоровье в связи с использованием «гормонов».
Среди наиболее часто встречающихся местных нежелательных реакций ИГКС следует отметить ротоглоточный кандидоз (реже – кандидоз пищевода), осиплость голоса, раздражение верхних дыхательных путей, вызывающее кашель и бронхоспазм. В связи с этим примерно 1/3 больных с легкой астмой настроена на применение препаратов «по потребности», т.е. только при появлении симптомов [7].
Меры профилактики возможных побочных эффектов ИГКС включают использование минимально необходимой дозы и сокращение кратности их приема, правильную ингаляционную технику, полоскание рта после ингаляции, нормализацию физической активности, отказ от вредных привычек.
Дополнительным классом противовоспалительных средств, используемых при БА, выступают АЛТ. Среди них в нашей стране у взрослых и детей в настоящее время применяется блокатор рецептора цистенил-лейкотриенов монтелукаст. Он уменьшает выраженность симптомов и частоту обострений астмы, бронхиальную гиперреактивность и хроническое воспаление дыхательных путей. АЛТ показаны для поддерживающего терапии БА, но их противовоспалительный эффект выражен слабее, чем у ИГКС. Монтелукаст выпускается в таблетках, что способствует большей приверженности к лечению у пациентов, которые в силу разных причин не желают или не могут применять ингаляционную терапию. Кроме того, он обладает хорошей переносимостью. АЛТ могут применяться на различных ступенях лечения, а также при аспириновой астме, БА физического усилия и аллергическом рините [2, 8].
Кромоны – кромогликат натрия и недокромил натрия – стабилизируют мембраны тучных клеток и тем самым подавляют выделение медиаторов в раннюю и позднюю фазу аллергических реакций. По силе противовоспалительного эффекта эти препараты значительно уступают ИГКС, в связи с чем в современных рекомендациях не входят в перечень препаратов, рекомендуемых для ступенчатой терапии астмы [2].
ФАРМАКОТЕРАПИЯ ЛЕГКОЙ БРОНХИАЛЬНОЙ АСТМЫ
Наибольшие изменения в международных и национальных рекомендациях произошли в концепции ведения пациентов с легких течением БА. Во-первых, они касаются использования единого подхода к лечению этого заболевания независимо от частоты симптомов (интермиттирующей или постоянной), во-вторых, исключения монотерапии КДБА, в том числе и при редких проявлениях болезни.
Предпочтительным режимом лечения легкой БА служит ситуационное использование комбинации ИГКС/формотерол вместо КДБА. Такая схема терапии позволяет решить одну из самых серьезных проблем ведения пациентов – низкую приверженность к терапии ИГКС. Она предусматривает возможность гибко варьировать дозу этих препаратов в зависимости от выраженности симптомов в конкретный момент и позволяет уменьшить частоту обострений астмы.
Новые рекомендации по лечению легкой БА основаны на результатах масштабных исследований по применению фиксированной комбинации будесонид + формотерол в режиме «по требованию» – SYGMA 1 и 2 (SYmbicort Given as need in Mild Asthma) [9, 10]. В них было установлено, что в плане достижения контроля астмы и снижения обострений применение комбинации будесонид/формотерол «по потребности» было эффективнее, чем ситуационный прием КДБА тербуталина, и не отличалось от постоянного приема будесонида по влиянию на количество обострений. Показано, что кумулятивная доза будесонида при его использовании в фиксированной комбинации с формотеролом «по требованию» была на 83% ниже, чем при постоянном применении этого ИГКС.
Эти результаты были подтверждены в открытых исследованиях (NovelSTART, PRACTICAL), выполненных в условиях реальной клинической практики и предусматривавших включение большого числа пациентов с легкой БА [11, 12].
В настоящее время четко не определена частота ситуационного применения комбинации ИГКС/формотерол при хорошем контроле астмы. По мнению экспертов GINA, к группе с контролируемым течением заболевания можно отнести пациентов, использующих ингалятор «по потребности» менее 2 раз/нед и соответствующих другим критериям контроля [2].
Другой возможностью лечения легкой БА является применение АЛТ. Они могут назначаться, например, при сочетании астмы и аллергического ринита, а также в случаях предпочтения такой терапии самими пациентами [8].
ФАРМАКОТЕРАПИИ БА СРЕДНЕЙ ТЯЖЕСТИ ТЕЧЕНИЯ
Астма средней степени тяжести предусматривает достижение контроля при применении 3-й ступени терапии. Как предпочтительный вариант лечения в этом случае рассматривается использование низких доз комбинации ИГКС/формотерол для поддерживающего лечения и «по требованию» для купирования симптомов (терапия единым ингалятором). Второй вариант – постоянное применение комбинации низких доз ИГКС/ДДБА и дополнительные ингаляции КДБА «по потребности» (см. рис.).
Эффективность режима единого ингалятора была доказана в многочисленных клинических исследованиях и наблюдениях в реальной клинической практике. Подобный режим дозирования позволяет предотвратить тяжелые обострения БА, снизить частоту госпитализаций пациентов и улучшить контроль астмы при использовании более низких доз ИГКС [13].
Режим единого ингалятора для применения у взрослых и подростков 12 лет и старше зарегистрирован в России для препаратов состава будесонид/формотерол в форме порошкового ингалятора (ДПИ) и беклометазона дипропионат/формотерол в виде дозированного аэрозольного ингалятора (ДАИ) [3].
Другие варианты 3-й ступени терапии БА – регулярное применение средних доз ИГКС, что является менее эффективным методом, чем добавление к терапии ДДБА, а также регулярное использование низких доз ИГКС и АЛТ с ситуационным введением («по потребности») КДБА [2].
ФАРМАКОТЕРАПИЯ ТЯЖЕЛОЙ АСТМЫ
У пациентов с тяжелой БА, кроме высоких доз комбинированных препаратов ИГКС + ДДБА, могут применяться ДДХЛ, биологические препараты, системные ГКС, макролиды и бронхиальная термопластика.
Из длительно действующих холинолитиков (ДДХЛ) в качестве дополнения к ИГКС/ДДБА возможно использование монопрепаратов тиотропия бромида. Другой вариант – применение фиксированных комбинаций ДДХЛ/ДДБА/ИГКС (умеклидиния бромид/вилантерол/флутиказона фуроат, гликопиррония бромид/формотерол/белкометазона пропионат, гликопиррония бромид/индакатерол/ мометазон). Показано, что такая терапия улучшает функцию легких и контроль заболевания, а также снижает число обострений тяжелой БА [14–16].
Введение моноклональных антител, направленных против основных цитокинов, участвующих в формировании БА, является новым направлением в лечении астмы и требует фенотипирования пациентов на основании клинических критериев и биологических маркеров. Тщательный отбор в условиях специализированного центра необходим, во-первых, для получения максимального эффекта терапии, а во-вторых, для уменьшения неоправданных затрат, поскольку стоимость лечения иммунобиологическими препаратами высока.
При Т2-эндотипе заболевания используются гуманизированные моноклональные антитела к IgE (омализумаб), интерлейкину (ИЛ)-5 (меполизумаб, реслизумаб) и рецепторам ИЛ-5 (бенрализумаб), полностью человеческое антитело к альфа-цепи рецептора ИЛ-4 (анти-ИЛ-4/13, дупилумаб). Все они внесены в ступенчатую терапию БА [2].
Препараты моноклональных антител для лечения тяжелой эозинофильной астмы (табл. 3) уменьшают частоту обострений, улучшают контроль заболевания и качество жизни пациентов, повышают показатели функции легких, а также снижают потребность в системных ГКС у больных гормонозависимой БА [17–21]. В последние годы предложены алгоритмы дифференцированного назначения анти-IgE и анти-ИЛ-5 [22, 23].
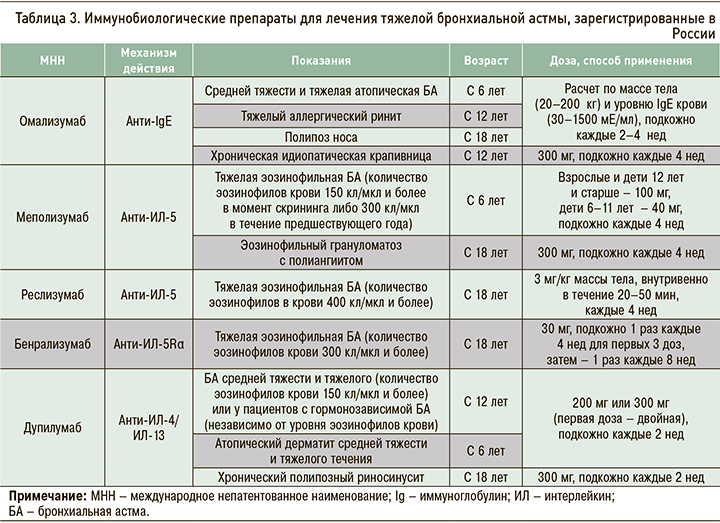
Другие показания (см. табл. 3) для назначения иммунобиологических препаратов включают тяжелый аллергический ринит (омализумаб), назальный полипоз (дупилумаб, омализумаб), атопический дерматит (дупилумаб), хроническую идиопатическую крапивницу (омализумаб), эозинофильный грануломатоз с полиангиитом (меполизумаб).
В последние годы исследователи открывают новые биологические молекулы, способные положительно воздействовать на течение БА: антагонисты тимического стромального лимфопоэтина (анти-TSLP), ИЛ-33, рецептора ИЛ-33 и др. [24– 26]. Проводятся исследования «малых молекул», предназначенных для ингаляционного введения.
Поддерживающее лечение пероральными ГКС используется у пациентов с тяжелой БА в отсутствие эффекта высоких доз ИГКС и недоступности/неэффективности иммунобиологических препаратов. Применяются минимальные дозы пероральных ГКС (преднизолон ≤7,5 мг/сут или эквивалентная доза другого стероида), позволяющие достичь контроля астмы. Недостаток такой терапии заключается в многочисленных побочных эффектах. В связи с наличием ИГКС и внедрением в клиническую практику принципиально новой биологической терапии БА длительное назначение пероральных ГКС в настоящее время применяется достаточно редко.
В качестве других методов лечения тяжелой БА (эозинофильного и неэозинофильного фенотипа) рекомендуется длительный прием азитромицина (500 мг 3 раза/нед в течение не менее 6 мес) в связи с наличием у него противовоспалительной и иммуномодулирующей активности. Показано, что длительные курсы этого антибактериального средства снижают частоту обострений астмы [27]. Данный подход не рекомендуется для рутинной клинической практики в связи с возможным развитием резистентности микрофлоры и развитием побочных эффектов (снижение слуха и др.).
Для лечения тяжелой резистентной БА также предложена бронхиальная термопластика, механизм действия которой основан на снижении массы гладких мышц дыхательных путей путем нагревания бронхиальной ткани до 65 °C с помощью радиочастотного катетера, вводимого через бронхоскоп. При ее использовании у пациентов уменьшалась частота обострений БА и улучшалось качество жизни, при этом контроль заболевания существенно не изменялся [28].
В 2018–2020 гг. на кафедре пульмонологии Северо-Западного государственного медицинского университета им. И.И. Мечникова под наблюдением находились 444 пациента в возрасте от 18 до 90 лет: 126 человек с легкой БА, 158 – средней тяжести и 160 – с тяжелой. Они были направлены на консультацию врачами общей практики и терапевтами. Среди этих пациентов терапию ИГКС НЕ получали 79% человек с астмой легкого течения, 49% со средней тяжестью заболевания и 16% с тяжелой БА (они либо не выполняли назначения врача, либо этот класс препаратов не был им назначен врачом). После консультации 1-я ступень терапии была рекомендована 7% пациентов, 2-я – 14%, 3-я – 40%, 4-я – 23%, 5-я – 16%. На 5-й ступени лечения у 30 больных тяжелой астмой до направления на консультацию использовались пероральные ГКС, у 59 пациентов применялась терапия иммунобиологическими препаратами (омализумабом, меполизумабом, бенрализумабом, дупилумабом). Следует отметить, что 7 больным БА средней степени тяжести также назначалась биологическая терапия с учетом сопутствующих Т2-зависимых заболеваний (полипозного риносинусита, атопического дерматита), которые являлись показаниями к данному виду лечения. При тяжелой астме исходно неконтролируемые симптомы (средний балл вопросника ACQ-5 ≥1,5) заболевания имели 80% больных на 4-й ступени и 78% – на 5-й. Через год проводимого лечения доля пациентов с неконтролируемой БА на 4-й ступени снизилась до 57% (p <0,01), на 5-й ступени – до 58% (p <0,05). Клинически значимая разница в улучшении контроля астмы (снижение среднего балла ACQ-5 на ≥0,5) через год лечения была достигнута у 51% больных тяжелой БА. У 12 пациентов удалось полностью отменить пероральные ГКС, у 10 больных – значительно снизить их дозу.
Полученные результаты подтверждают, что ведение пациентов с БА в условиях первичного звена здравоохранения нередко не соответствует национальным и международным клиническим рекомендациям. При использовании современных подходов к лечению контроль заболевания может быть достигнут у значительной части больных тяжелой БА.
АЛЛЕРГЕН-СПЕЦИФИЧЕСКАЯ ИММУНОТЕРАПИЯ
Аллерген-специфическая иммунотерапия (АСИТ) заключается в повторных введениях пациентам с аллергической астмой лечебных аллергенов с целью снижения чувствительности к их воздействию. Классический способ иммунотерапии представляет собой подкожные инъекции стандартизованных водно-солевых растворов аллергенов (клещевых, пыльцевых, грибковых, эпидермальных аллергенов и аллергенов яда насекомых) в постепенно возрастающих концентрациях по специальным схемам и далее поддерживающее лечение в течение 3–5 лет. В последние годы в России и разных зарубежных странах начала активно использоваться сублингвальная АСИТ. Показано, что она улучшает контроль, уменьшает потребность больных в ИГКС и частоту обострений астмы [29, 30]. Полученные результаты позволили включить АСИТ в схему ступенчатой терапии (см. рис.) [2].
Этот метод, впрочем, имеет ряд ограничений. АСИТ противопоказана при обострениях астмы, отсутствии контроля и тяжелом течении заболевания (ОФВ1 <70% на фоне адекватной терапии), множественной сенсибилизации (более 3 аллергенов), наличии ряда тяжелых фоновых заболеваний, низкой дисциплине больных, т.е. АСИТ показана только части пациентов – взрослым и детям с аллергической астмой легкого и средней тяжести течения.
ЗАКЛЮЧЕНИЕ
Таким образом, современная терапия позволяет достичь контроля течения БА у значительной доли пациентов. Большое значение имеет осведомленность врачей различных специальностей о подходах к лечению этого заболевания. Внедрение в клиническую практику международных и национальных рекомендаций, несомненно, улучшит качество ведения пациентов и снизит вероятность неблагоприятных исходов при этом заболевании.



