ВВЕДЕНИЕ
Хроническая обструктивная болезнь легких (ХОБЛ) представляет собой глобальную проблему современной медицины, ассоциированную с сердечно-сосудистыми заболеваниями [1]. В основе тесной взаимосвязи ХОБЛ и сердечно-сосудистой патологии лежит ряд единых патогенетических механизмов, таких как гипоксемия, легочная гипертензия, системное воспаление, окислительный стресс, общие факторы риска [2]. Наличие коморбидных кардиоваскулярных заболеваний у больных ХОБЛ может привести к неблагоприятным исходам, начиная от ухудшения качества жизни и заканчивая повышением общей и сердечно-сосудистой смертности [2]. У пациентов с ХОБЛ возрастает вероятность развития ишемической болезни сердца (ИБС), аритмий, сердечной недостаточности, заболеваний периферических сосудов [3].
Пациенты с кардиореспираторной патологией имеют значимо повышенные показатели системного воспаления (С-реактивный белок, прокальцитонин, фибриноген) [4]. К независимым предикторам летальности у таких пациентов относятся интенсивность курения, возраст, повышение уровня С-реактивного белка и фибриногена, а также результат 6-минутного теста-ходьбы [5]. В настоящее время ведется поиск маркеров, позволяющих прогнозировать риск сердечно-сосудистых осложнений у пациентов с ХОБЛ. В литературе имеются данные о cвязи матриксных металлопротеиназ, мозгового натрийуретического пептида, интерлейкина 6 (ИЛ-6), ИЛ-8 с повышением частоты ранних неблагоприятных исходов, сердечно-сосудистой заболеваемости и смертности [6–8]. Одним из описанных в литературе маркеров системного воспаления является остеопонтин: было выявлено, что повышение его уровня связанно с риском развития неблагоприятных сердечно-сосудистых событий [9–11]. Кроме того, проведены исследования, в которых диагностировано повышение уровня ОПН в образцах мокроты пациентов с ХОБЛ и показана тесная взаимосвязь повышенного уровня этого маркера с обострением ХОБЛ [12, 13].
В настоящее время проблема поиска предиктора прогрессирования ХОБЛ и развития осложнений крайне актуальна, ведь определение такого маркера позволило бы совершенствовать подходы в тактике ведения и в фармакологической терапии пациентов с ХОБЛ. В научной литературе отсутствуют данные о взаимосвязи уровня ОПН с частотой сердечно-сосудистых событий у пациентов с ХОБЛ. В связи с этим целью нашего исследования стала оценка влияния повышенного уровня остеопонтина (ОПН) на частоту госпитализаций по поводу обострения ХОБЛ и частоту сердечно-сосудистых событий у пациентов с кардиореспираторной патологией.
МАТЕРИАЛ И МЕТОДЫ
Исследование было проведено на кафедре госпитальной терапии им. академика П.Е. Лукомского лечебного факультета ФГАОУ ВО «Российский национальный исследовательский медицинский университет им. Н.И. Пирогова» Минздрава России на базе ГБУЗ «Городская клиническая больница № 15 им. О.М. Филатова Департамента здравоохранения г. Москвы».
В исследование было включено 99 пациентов мужского и женского пола старше 18 лет с установленным диагнозом ХОБЛ A–D по шкале GOLD в сочетании с ИБС и без ИБC. Диагноз ХОБЛ устанавливался в соответствии с рекомендациями GOLD [1]. Диагноз ИБС у всех пациентов был верифицирован ранее, согласно рекомендациям Российского кардиологического общества и МКБ- 10, о чем свидетельствовала подтвержденная медицинская документация об обращении за медицинской помощью в амбулаторном порядке или при стационарном лечении.
Участники исследования были разделены на две группы: первую составили 49 пациентов с диагнозом ХОБЛ в сочетании с ИБС (далее – 1-я группа, или «ИБС + ХОБЛ»), вторую – 50 пациентов с диагнозом ХОБЛ, но без ИБС (далее – 2-я группа, или «ХОБЛ без ИБС»).
Дизайн исследования включал следующие процедуры:
1-й этап – проведение скрининга;
2-й этап – распределение пациентов по группам, оценка статуса курения, объективный осмотр, оценка медикаментозной терапии, лабораторное обследование (исследование уровня ОПН с помощью наборов Platinum ELISA (Bender MedSystems, Австрия)), инструментальное обследование (спирометрия с бронходилатационной пробой), выполнение 6-минутного теста ходьбы, определение индекса BODE, заполнение опросников CAT и mMRC, оценка сердечно-сосудистого риска по шкале SCORE;
3-й этап – телефонный контакт через 12 мес для оценки частоты госпитализации по поводу ХОБЛ, госпитализации по поводу сердечно-сосудистых событий.
Концентрация ОПН в исследовании рассчитывалась путем построения калибровочной кривой с помощью компьютерной программы и выражалась в нг/мл.
Статистический анализ проводился с использованием программы IBM SPSS Statistics v.25 (разработчик — IBM Corporation). Статистически значимыми считали значения р value <0,05. Значения коэффициента корреляции ρ интерпретировались в соответствии со шкалой Чеддока. Построение прогностической модели риска определенного исхода выполнялось при помощи метода бинарной логистической регрессии. Для оценки диагностической значимости количественных признаков при прогнозировании определенного исхода, в том числе вероятности наступления исхода, рассчитанной с помощью регрессионной модели, применялся метод анализа ROC-кривых.
Исследование было одобрено этическим комитетом ФГАОУ ВО «Российский национальный исследовательский медицинский университет им. Н.И. Пирогова» Минздрава России. Включение пациентов в исследование осуществлялось после подписания формы добровольного информированного согласия.
РЕЗУЛЬТАТЫ
В анализ частоты госпитализаций по поводу сердечно-сосудистых событий и обострения ХОБЛ за год наблюдения было включено 48 пациентов из 1-й группы и 49 из 2-й группы. В конечный анализ не были включены 1 пациент из группы «ИБС + ХОБЛ» и 1 пациент из группы «ХОБЛ без ИБС» в связи с наступлением летального исхода в период текущей госпитализации после рандомизации больных.
При анализе частоты госпитализаций по поводу обострения ХОБЛ в 1-й группе доля пациентов без таких госпитализаций составила 58,3% (n=28), с одной госпитализацией за период наблюдения – 37,5% (n=18), с двумя – 4,2% (n=2); во 2-й группе аналогичные показатели равнялись 73,5% (n=37), 20,4% (n=10) и 6,1% (n=3) соответственно. Различия между группами были статистически незначимы (р=0,121; рис. 1).

При анализе частоты госпитализаций по поводу сердечно-сосудистых событий было выявлено, что в 1-й группе доля пациентов без таких госпитализаций составила 54,2% (n=26), с одной госпитализацией – 39,6% (n=19), с двумя – 4,2% (n=2), с тремя – 2,0% (n=1). Во 2-й группе не было зафиксировано случаев двух или трех госпитализаций по поводу сердечно-сосудистых событий за период наблюдения; у 96,0% (n=47) пациентов этой группы такие госпитализации отсутствовали вовсе, у 4,0% (n=2) произошла одна госпитализация. Различия между группами по частоте сердечно-сосудистых событий оказались статистически значимыми (р <0,001; рис. 2).
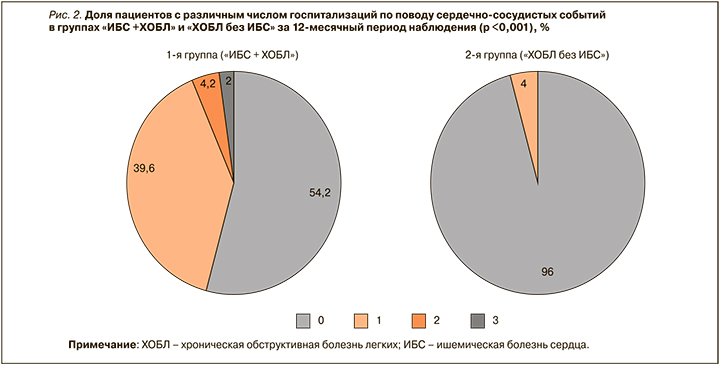
В структуру госпитализаций по поводу сердечно-сосудистых событий вошли декомпенсация хронической сердечной недостаточности (n=17), острый инфаркт миокарда (n=3), острое нарушение мозгового кровообращения (n=2), пароксизм фибрилляции предсердий (n=3), гипертонический криз (n=1), стенокардия напряжения (n=2), нестабильная стенокардия (n=1).
При проведении корреляционного анализа в общей группе пациентов была выявлена слабая положительная взаимосвязь между частотой госпитализаций по поводу обострения ХОБЛ и шкалой GOLD (ρ=0,266, p=0,008), умеренная положительная корреляционная взаимосвязь с индексом BODE (ρ=0,331, p <0,001), слабая прямая корреляционная взаимосвязь со шкалой САТ (ρ=0,240, p=0,017), умеренная положительная корреляционная взаимосвязь со шкалой mMRC (ρ=0,330, p <0,001), слабая прямая корреляционная взаимосвязь с уровнем С-реактивного белка (ρ=0,208, p=0,05), слабая отрицательная корреляционная взаимосвязь с показателем ОФВ1 (ρ=-0,166, p=0,101).
При выполнении регрессионного анализа в общей группе пациентов была обнаружена прямая слабая корреляционная взаимосвязь уровня ОПН с показателями частоты госпитализаций по поводу сердечно-сосудистых событий (ρ=0,234, p=0,020) и по поводу ХОБЛ (ρ=0,093, p=0,30). В группе «ИБС + ХОБЛ» отмечалась слабая положительная корреляционная взаимосвязь между частотой госпитализацией по поводу сердечно-сосудистых событий и уровнем ОПН (ρ=0,208, p=0,152). В группе «ХОБЛ без ИБС» была установлена умеренная положительная корреляционная взаимосвязь между уровнем ОПН и частотой госпитализаций по поводу обострения ХОБЛ (ρ=0,305, p=0,031), а также слабая положительная корреляционная взаимосвязь между уровнем этого маркера и частотой госпитализаций по поводу сердечно-сосудистых событий (ρ=0,078, p=0,591). Учитывая полученные данные, нами была разработана программа с использованием системы прогнозирования для выделения группы риска повышения частоты госпитализаций (по поводу обострения ХОБЛ или сердечно-сосудистых событий; табл. 1). Исходя из значений регрессионных коэффициентов, ОПН имеет прямую связь с вероятностью развития госпитализации по поводу обострения ХОБЛ или сердечно-сосудистого события. Сводка для модели представлена в таблице 2.

Наблюдаемая зависимость описывается уравнением:
p = 1/ (1 + e-z) × 100%
z = -0,902 + 0,012 × X,
где p – вероятность наступления события; X – значение уровня ОПН; z – уравнение регрессии.
Исходя из значения коэффициента детерминации Найджелкерка, модель (см. табл. 2) учитывает вероятность развития госпитализации от уровня ОПН в 8,5% случаях.
Полученные результаты были подвергнуты ROC-анализу, на основании которого были построены соответствующие ROC-кривые. В разработанной прогностической модели площадь под ROC-кривой составила 0,652±0,055 (95% доверительный интервал (ДИ): 0,543–0,760), что свидетельствует о статистически значимой прогностической способности этой модели (рис. 3, табл. 3).

Пороговое значение функции в точке cut-off составляло 71,74 (чувствительность – 60,4%, специфичность – 60,8%), т.е. значения уровня ОПН 71,74 нг/мл и выше соответствовали наличию госпитализации по поводу обострения ХОБЛ или сердечно-сосудистого события.
ОБСУЖДЕНИЕ
Целями оценки тяжести ХОБЛ является определение степени ограничения воздушного потока, его влияния на состояние здоровья пациента и риск обострения. В настоящее время инструментами для оценки тяжести ХОБЛ служат шкалы CAT, mMRC, показатель ОФВ1 [14]. Можно предположить, что индекс BODE не только определяет 4-летнюю выживаемость пациентов с ХОБЛ [14], но его повышение способно также косвенно указывать на повышенный риск госпитализации по поводу обострения заболевания. Частота обострений ХОБЛ относится к известным предикторам прогрессирования заболевания [14], в связи с чем важно учитывать имеющиеся сведения о серьезных обострениях и принимать во внимание потенциальное влияние сопутствующих состояний на риск усугубления ХОБЛ.
Наши результаты подтверждают представление о том, что наличие сопутствующей патологии, в частности сердечно-сосудистой, связано с риском частых обострений ХОБЛ, требующих госпитализации пациента [15]. Частота госпитализаций по поводу сердечно-сосудистых событий в группе пациентов с ИБС и ХОБЛ было достоверно выше, по сравнению с группой пациентов с ХОБЛ, но без ИБС. Основную роль в структуре таких госпитализаций, согласно нашим результатам, играет хроническая сердечная недостаточность, что также находит свое подтверждение в литературе [15].
Определение биомаркеров в крови, в частности ОПН, может помочь в прогнозировании обострения ХОБЛ. ОПН достоверно связан с частотой госпитализаций у пациентов с ХОБЛ и ИБС. Из описанных в литературе маркеров пока лишь декорин и альфа-2-макроглобулин показали прогностическую ценность в определении будущих тяжелых обострений и госпитализаций [16]. При уровне ОПН выше 71,74 нг/ мл необходимо выделять группу повышенного риска среди данной когорты больных и уделять особое внимание соблюдению рекомендаций для снижения риска госпитализации по поводу сердечно-сосудистых событий и обострения ХОБЛ.
Недостатками нашего исследования явился ограниченный объем выборки, наблюдательный характер исследования.
ЗАКЛЮЧЕНИЕ
По результатам нашего исследования выявлено, что уровень ОПН имеет достоверную прямую связь с вероятностью развития госпитализации по поводу обострения ХОБЛ или сердечно-сосудистого события у пациентов в группе «ИБС+ ХОБЛ» и в группе «ХОБЛ без ИБС». Определение уровня ОПН способно помочь в совершенствовании подходов к ведению пациентов с ХОБЛ, выделении групп риска, проведении своевременной коррекции терапии.



