ВВЕДЕНИЕ
Венозная тромбоэмболия (ВТЭ), включающая тромбоз глубоких вен (ТГВ) и тромбоэмболию легочной артерии (ТЭЛА), – важная причина заболеваемости и смертности среди пациентов с онкологическими заболеваниями [1]. Онкологический процесс влияет на систему гемостаза, что может проявляться у разных пациентов склонностью как к тромбозам, так и к кровотечениям. У пациентов с раком вероятность развития ВТЭ значительно выше, чем у людей без онкологической патологии [2, 3], при этом наблюдается более высокая частота рецидивов ВТЭ и геморрагических осложнений во время ее лечения [4]. ВТЭ относят к наиболее частым и опасным осложнениям, которые развиваются не менее чем у 4–20% онкологических пациентов и становятся одной из основных причин их смерти [5].
Фармакологическая тромбопрофилактика ВТЭ проводится большинству госпитализированных онкологических больных с острым заболеванием и пациентам, перенесшим серьезную операцию по удалению опухоли. В то же время роль такой тромбопрофилактики в амбулаторных условиях остается неясной. Амбулаторные онкологические пациенты, получающие системную противораковую терапию, подвергаются различному риску ВТЭ, который зависит от типа рака, стадии опухолевого процесса, вида противоракового лечения и индивидуальных факторов риска пациента. К локализациям с самым высоким риском венозных тромбоэмболических осложнений (ВТЭО) в случае рака относят поджелудочную железу, почки, яичники, легкие и желудок. Иногда развитие венозного тромбоза (особенно у пациентов без дополнительных факторов риска развития ВТЭ) может быть первым признаком недиагностированного рака. ВТЭ у онкологических пациентов может ухудшать качество и сокращать срок жизни, отсрочивать хирургическое лечение, препятствовать проведению химиотерапии, а также увеличивать вероятность рецидива рака и ранней смертности [6]. В связи с этим на первый план выходят стратегии тромбопрофилактики у онкологических пациентов.
Первичная тромбопрофилактика (т. е. еще до развития первого сердечно-сосудистого события) обычно назначается пациентам в специализированных лечебно-профилактических учреждениях и остается зоной ответственности онкологов. В то же время она может быть предложена и амбулаторным больным высокого риска с онкологическими заболеваниями, например при прогрессирующей карциноме поджелудочной железы, герминогенной опухоли яичка с метастазами в забрюшинные лимфоузлы размером свыше 3,5 см, местно-распространенном или метастатическом раке поджелудочной железы/легких в отсутствие факторов риска кровотечения и нежелательных лекарственных взаимодействий.
Для своевременного выявления пациентов с онкологическими заболеваниями, имеющих высокий риск ВТЭ, и проведения целенаправленной фармакологической профилактики тромбоза более 15 лет назад Khorana A.A. et al. разработали объективную и стандартизированную модель прогнозирования такого риска, называемую в честь автора шкалой Хорана [7]. Основанная на типе опухоли, индексе массы тела и трех лабораторных параметрах, она позволяет стратифицировать пациентов с опухолями в амбулаторных условиях в зависимости от риска ВТЭ. Валидационные исследования указывают на то, что у больных с показателем ≥ 2 баллов по шкале Хорана риск ВТЭ составляет 9,6%.
Действующие российские клинические рекомендации и различные международные руководства различаются в вопросах выбора антикоагулянта для тромбопрофилактики при онкологических заболеваниях. Например, если в соответствующем действующем руководстве Германии по-прежнему отдается предпочтение низкомолекулярным гепаринам (НМГ) перед прямыми оральными антикоагулянтами (ПОАК) [8], то гайдлайны Международного общества по тромбозу и гемостазу (International Society on Thrombosis and Hemostasis, ISTH) предлагают использовать у амбулаторных онкологических больных высокого риска именно ПОАК при условии отсутствия лекарственных взаимодействий и высокого риска кровотечения [9]. Отметим, что ни один ПОАК в настоящее время не зарегистрирован по этому показанию.
Еще сложнее обстоит дело с вторичной тромбопрофилактикой у онкологических пациентов (т. е. профилактикой повторных тромбозов). Частота повторных ВТЭ у больных со злокачественными новообразованиями в течение первого года лечения достигает 20,7%. При этом количество серьезных геморрагических осложнений также выше, чем в среднем по популяции [4].
С учетом маршрутизации при выписке на амбулаторное лечение после первого эпизода ТГВ или ТЭЛА динамическое наблюдение за пациентами, принимающими антикоагулянты, часто осуществляют участковые терапевты. Следует иметь в виду, что в отличие от общей популяции у пациента с установленным активным раком и наличием ТГВ/ТЭЛА одним из критериев прекращения антикоагулянтной терапии по истечении 6 мес. является купирование онкологического процесса с последующей оценкой баланса риска и пользы от продления антикоагуляции.
ВТЭО обходятся системе здравоохранения дорого, поскольку соответствующие пациенты нуждаются в длительной антикоагулянтной терапии и наблюдении в течение минимум 6 мес. после лечения. При повторном эпизоде ТГВ или ТЭЛА на фоне существующего онкологического процесса, как правило, необходима пожизненная антикоагулянтная терапия. Увеличение времени ее проведения повышает как финансовую, так и ресурсную нагрузку на систему здравоохранения, поскольку больным требуются дополнительное наблюдение и обследования, чтобы убедиться, что ВТЭ не прогрессирует.
Согласно рекомендациям российских экспертов по профилактике, диагностике и лечению ТГВ, при выборе лекарственного средства (ЛС) для длительной терапии у пациентов с онкологическими заболеваниями и ТГВ/ТЭЛА необходимо отдавать приоритет оральным ингибиторам Xа-фактора (апиксабану, ривароксабану, эдоксабану); на втором месте находится ингибитор тромбина дабигатрана этексилат, на третьем – НМГ далтепарин натрия (рис. 1). Антагонисты витамина К (варфарин) для продолжительного применения у этой группы пациентов не рекомендованы [10].
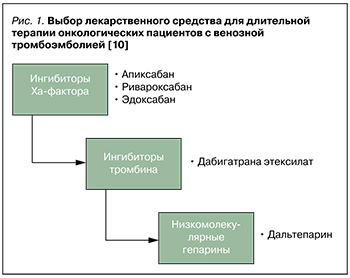
Несмотря на то что НМГ обеспечивают достоверное снижение риска рецидива ВТЭ, их использование на амбулаторном этапе по целому ряду причин не идеальный вариант тромбопрофилактики. Так, к недостаткам этой группы препаратов можно отнести местные реакции при введении, боль при аутоинъекции, появление синяков, кровотечения, тошноту, рвоту, а также стоимость ЛС.
Отдельно следует отметить, что уровень приверженности амбулаторной тромбопрофилактике у пациентов с онкологическими заболеваниями точно не известен. При этом данные литературы по ортопедии свидетельствуют, что среди ревматологических больных этот уровень составляет всего около 60% [13, 14].
Учитывая необходимость длительной тромбопрофилактики, оценка экономической эффективности использования у целевых пациентов различных терапевтических тактик представляет особый интерес. Целью данного исследования стал фармакоэкономический анализ обоснованности применения лекарственного препарата Эликвис® (апиксабан) для вторичной профилактики ТГВ и ТЭЛА у пациентов с онкологическими заболеваниями по сравнению с другими пероральными ЛС.
МАТЕРИАЛ И МЕТОДЫ
Экономическая оценка проведена с позиции интересов здравоохранения Российской Федерации. Все виды клинико-экономического анализа выполнены в соответствии с отраслевым стандартом «Клинико-экономические исследования», применяемым в нашей стране. Проведены анализ прямых затрат в системе здравоохранения, анализ «затраты – эффективность» и анализ чувствительности.
Критерием эффективности тромбопрофилактики выступил комбинированный показатель частоты развития ишемического инсульта (ИИ), системной эмболии и выживаемости пациентов, критерием безопасности – показатель частоты развития тяжелых кровотечений (ТК) и клинически значимых нетяжелых кровотечений (КЗНК). Оценка осуществлялась на основании данных ранее выполненных метаанализов, результаты которых приведены в доступных публикациях. Суммарные расходы на ведение пациента для каждой из сравниваемых альтернативных тактик лечения определялись с помощью расчета стоимости лекарственной терапии, а также затрат на купирование нежелательных явлений в течение 12 мес. Вывод о наиболее предпочтительном варианте терапии делался в соответствии с данными о соотношении ее эффективности и стоимости.
При расчете учитывались суточные дозы апиксабана, дабигатрана этексилата и ривароксабана. К частным случаям анализа «затраты – эффективность» относят анализ минимизации затрат, применяемый в отсутствие статистически значимых различий эффективности сравниваемых альтернативных методов лечения, когда предпочтение отдается более экономичной тактике терапии. Показатель минимизации затрат вычислялся по формуле:
CMA = Cost1 - Cost2,
где CMA – показатель, Cost1 – прямые затраты на одного пациента при использовании апиксабана, Cost2 – прямые затраты на одного пациента при использовании альтернативной тактики.
В ходе исследования выполнялся анализ научной литературы по рассматриваемой теме. В базах данных PubMed (MEDLINE), Scopus, eLibrary и «Киберленинка» делались поисковые запросы по ключевым словам, включавшие названия ЛС или общего названия группы антикоагулянтных препаратов (*apixaban (Eliquis®), *dabigatran (Pradaxa®), *rivaroxaban (Xarelto®), *non-vitamin K antagonist oral anticoagulants, *new oral anticoagulants, *novel oral anticoagulants, *direct oral anticoagulants, *direct-acting oral anticoagulants), а также слов, описывающих показания к их применению с целью профилактики и лечения у пациентов с ВТЭО (*pulmonary embolism, *venous thromboembolism, *deep vein thrombosis, *thrombosis). В базах данных, содержащих информацию на русском языке, запрос составляли по такому же алгоритму, с использованием аналогичных русскоязычных терминов.
Структура моделей
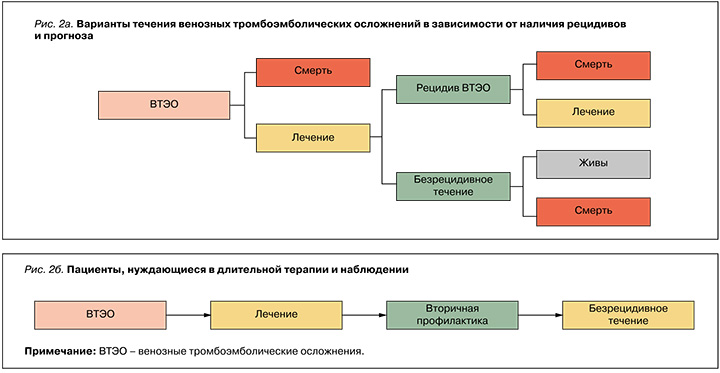
С учетом особенностей основного заболевания риск рецидива после первого тромбоэмболического эпизода у онкологических пациентов выше, чем в среднем по популяции. В связи с этим нами была использована упрощенная схема фармакоэкономической модели тромбопрофилактики (рис. 2а), учитывавшая вторичную тромбопрофилактику (рис. 2б).
Структура затрат
Ранее мы анализировали фармакоэкономические аспекты лечения ВТЭ у пациентов с онкологическими заболеваниями. Было установлено, что наименьшие расходы на ведение пациента имели место при применении тактики лечения, основанной на приеме апиксабана, – 74 689 руб. В то же время суммарные затраты при использовании ривароксабана были на 22,5% выше и достигали 91 508 руб. в расчете на одного пациента. Сходные показатели были и в группе варфарина – 93 379 руб. на пациента, что на 25% выше затрат при терапии апиксабаном [15].
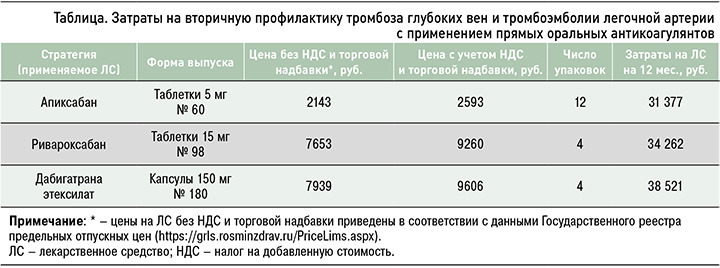
Для оценки стоимости вторичной профилактики в расчет брались прямые затраты – расходы на ЛС и купирование развившихся кровотечений. Структура затрат на ЛС представлена в таблице. Отметим, что в ней отсутствует эдоксабан, поскольку он, хотя и включен в отечественные клинические рекомендации и имеет регистрационное удостоверение в России, на момент проведения исследования отсутствовал в дистрибьюции; как следствие, установить прямые затраты при его использовании не было возможно. Для дабигатрана этексилата расходы на ЛС представлены как средняя величина от зарегистрированных стоимостей всех препаратов (торговых наименований, ТН) с этим МНН в форме выпуска «капсулы 150 мг № 180». Для ривароксабана – как средняя величина от зарегистрированных стоимостей всех препаратов (ТН) с этим МНН в форме выпуска «таблетки 15 мг № 98».
Затраты на купирование клинически значимых кровотечений были определены в соответствии с тарифом на оказание медицинской помощи по обязательному медицинскому страхованию. Расходы на купирование ТК составляют 125 691 руб., КЗНТК – 55 110 руб.
При построении фармакоэкономических моделей мы исходили из того, что пациент с онкологическим заболеванием получал антикоагулянт с целью вторичной профилактики ВТЭ и находился под динамическим наблюдением с безрецидивным течением процесса.
РЕЗУЛЬТАТЫ
Использованные нами данные об эффективности и безопасности антикоагулянтов основаны на результатах ранее выполненного метаанализа, который объединяет результаты специализированных рандомизированных исследований, посвященных оценке применения апиксабана, ривароксабана, эдоксабана в сравнении с далтепарином натрия по показанию онкоассоциированный тромбоз [16–19]. Этот метаанализ продемонстрировал снижение риска рецидива ВТЭО на 38–41% в случае приема ингибиторов фактора Xа при отсутствии их влияния на риск большого кровотечения и общей смертности. При этом риск развития небольших, но клинически значимых кровотечений был увеличен в 1,45–1,65 раза [20].
В исследовании реальной клинической практики было показано, что апиксабан (из-за благоприятного профиля метаболизма) в отличие от ривароксабана и НМГ эноксапарина натрия назначают более возрастным пациентам и больным с умеренным и значительным снижением почечной функции [21]. Следует также иметь в виду, что во всех исследованиях изучались эффективность и безопасность только полных лечебных доз апиксабана и ривароксабана, поэтому до появления новых данных не рекомендуется использование их редуцированных дозировок в рамках продленной терапии онкоассоциированного тромбоза.
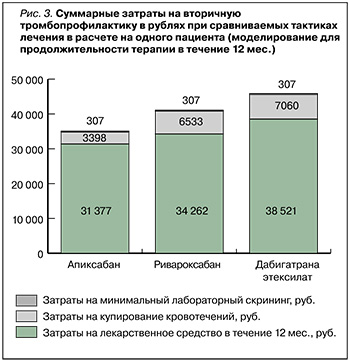
Согласно результатам моделирования эффектов 12-месячной антикоагулянтной терапии, наименьшие затраты на ЛС отмечались при применении тактики лечения, основанной на приеме апиксабана, – 31 377 руб. в расчете на одного пациента. Аналогичные расходы при терапии ривароксабаном составили 34 262 руб., а при приеме дабигатрана этексилата – 38 521 руб., что соответственно на 9,2 и 22,7% больше, чем при терапии апиксабаном.
Тактика, основанная на применении апиксабана, также обусловливала менее высокие затраты на купирование кровотечений – 3398 руб., в то время как при приеме ривароксабана они достигали 6533 руб. (выше в 1,9 раза), дабигатрана этексилата – 7060 руб. (выше в 2 раза).
Таким образом, суммарные расходы при терапевтической тактике с использованием апиксабана были минимальными и составили 35 082 руб., тогда как при применении ривароксабана эта величина равнялась 41 102 руб., дабигатрана этексилата – 45 888 руб. (рис. 3).
ОБСУЖДЕНИЕ
Вопрос вторичной тромбопрофилактики при онкологических патологиях остается во многом открытым. С уверенностью можно говорить, что рутинная фармакологическая тромбопрофилактика не должна назначаться всем амбулаторным больным с такими заболеваниями. Все пациенты на амбулаторном этапе должны быть стратифицированы по степени риска для выявления тех, у кого польза от профилактики венозной тромбоэмболии выше, чем угрозы, ассоциированные с кровотечениями.
Амбулаторным пациентам с онкологическими заболеваниями высокого риска (оценка по шкале Хорана 2 или выше) может быть предложена тромбопрофилактика апиксабаном, ривароксабаном или НМГ при условии отсутствия существенных факторов риска кровотечения и лекарственного взаимодействия. Рассмотрение такой терапии должно сопровождаться обсуждением с пациентом относительной пользы и вреда, стоимости препарата и продолжительности профилактики в данных условиях.
Риск развития рецидива ВТЭО и ТК значимо влияет на дополнительные затраты и качество жизни пациентов [22]. В связи с этим необходимо оценивать не только расходы на закупку лекарственных препаратов, но и влияние лечения на такие показатели, как качество жизни в целом, увеличение работоспособности и уровень повседневной активности [23].
С учетом представленных данных о сравнительной клинической эффективности различных вариантов антикоагулянтной терапии, а также о суммарных прямых затратах на вторичную профилактику развития ТГВ и ТЭЛА в популяции пациентов, страдающих онкологическими заболеваниями, тактика лечения, основанная на приеме апиксабана, представляется наиболее экономичной по сравнению с другими рассмотренными альтернативными тактиками. Исходя из этого, возможно уменьшить курсовую стоимость лечения и дополнительные расходы, связанные с развитием тяжелых и клинически значимых нетяжелых кровотечений.
Ограничения исследования. Исследование может иметь ограничения, связанные с изменениями тарифных соглашений территориальных фондов обязательного медицинского страхования, зарегистрированных предельных отпускных цен на лекарственные препараты, появления новых ЛС и обновлений в клинических рекомендациях, посвященных вопросам вторичной профилактики ВТЭО у пациентов с онкологическими заболеваниями.
ЗАКЛЮЧЕНИЕ
Результаты сравнения эффектов апиксабана с альтернативными ПОАК, доступными на территории РФ, при вторичной тромбопрофилактике на фоне онкологических заболеваний свидетельствуют о том, что применение этого ингибитора Ха-фактора может быть более обоснованным с учетом его преимущества по такому фармакоэкономическому показателю, как минимизация прямых затрат на проведение лекарственной терапии и купирование нежелательных реакций. У онкологических пациентов прием апиксабана для лечения и профилактики ТГВ и ТЭЛА также был более экономически эффективным в сопоставлении с другими антикоагулянтами по показателю «стоимость – эффективность терапии».



