В организме женщины во время беременности происходит гормональная перестройка в соответствии с меняющимися потребностями матери и развивающегося плода, именно в это время могут происходить гормональные перебои, способные привести к неблагоприятным исходам для матери и ребенка. По данным Всемирной организации здравоохранения (ВОЗ), в 2019 г. у 20 млн, или у 16%, женщин с естественными родами наблюдалась та или иная форма нарушения гликемии во время беременности, и 84% из них были вызваны гестационным сахарным диабетом (ГСД). Осложнения, обусловленные гестационным нарушением углеводного обмена, имел 1 из каждых 6 рожденных детей [1].
Распространенность ГСД у беременных колеблется от 1,7 до 20% с большими различиями в зависимости от страны, стратегии скрининга и диагностических критериев [2–3]. Известно, что заболеваемость сахарным диабетом у беременных увеличивается с возрастом матери, а также ассоциирована с высоким индексом массы тела (ИМТ) перед беременностью. Значимым фактором является и наследственная предрасположенность: известно, что при наличии семейного анамнеза диабета заболеваемость ГСД гораздо выше, нежели в его отсутствие [4–8]. У пациенток с плохо контролируемым сахарным диабетом риск потери беременности и развития других осложнений беременности и родов может возрастать до 50% [9]. ГСД не только увеличивает вероятность осложнений у матери и плода во время беременности, но и значительно увеличивает риск развития сахарного диабета 2 типа, метаболического синдрома (характеризующегося нарушенной толерантностью к глюкозе, центральным ожирением, дислипидемией и инсулинорезистентностью) и сердечно-сосудистых заболеваний после беременности [11–14].
В связи с вышесказанным женщинам с диагнозом ГСД рекомендуется проходить регулярный скрининг на предмет предиабета и сахарного диабета 2 типа, а также соблюдать основные принципы профилактики метаболических нарушений, основанных на модификации образа жизни, поддержании нормальной массы тела, расширении физической активности, сбалансированном рациональном питании [5, 10].
Наряду с нарушениями углеводного обмена одной из самых распространенных эндокринопатий у беременных является дисфункция щитовидной железы, преимущественно обусловленная снижением выработки тиреоидных гормонов. Гипотиреоз может быть причиной множества неблагоприятных исходов беременности и осложнений, связанных с потомством и беременностью [15, 16].
Распространенность гипотиреоза у женщин репродуктивного возраста составляет от 2 до 3%, а хронический аутоиммунный тиреоидит становится основной причиной гипотиреоза во время беременности. Высокая распространенность нарушений углеводного обмена и снижения функциональной активности щитовидной железы в период беременности объясняет высокий интерес специалистов и ученых к поиску возможной взаимосвязи этих патологий [17–21].
Щитовидная железа, как и все эндокринные органы, претерпевает существенные изменения во время беременности и выступает в этот период источником гормонов не только для матери, но и для развивающегося плода, в связи с чем потребность в тиреоидных гормонах в период гестации существенно возрастает. Недостаточная перестройка активности щитовидной железы может привести к развитию гипотиреоза, в структуре которого чаще встречается субклиническая форма. Известны многочисленные неблагоприятные последствия некомпенсированного гипотиреоза для течения беременности и развития плода. Тяжелый гипотиреоз у беременной женщины связан с повышенной угрозой неблагоприятных осложнений беременности, таких как антенатальная гибель плода, преждевременные роды, низкая масса тела при рождении, самопроизвольные выкидыши и гестационная гипертензия, а также может оказывать негативное воздействие на нейрокогнитивное развитие плода, что может привести к интеллектуальным нарушениям у ребенка [15, 16].
Актуальным предметом обсуждения на сегодняшний день является также вопрос о роли недостаточной функции щитовидной железы в развитии гестационных нарушений углеводного обмена. Так, существуют данные о том, что гипотиреоз отрицательно влияет на метаболизм глюкозы, ухудшая чувствительность тканей к инсулину и снижая утилизацию глюкозы тканями. При беременности гипотиреоз может быть связан с усугублением уже имеющейся физиологической инсулинорезистентности, развивающейся преимущественно со II триместра беременности, что может иметь значение в повышении риска развития ГСД у таких женщин [22–23]. В метаанализе Gong L.-L. et al., основанном на данных о 278 608 пациентах, было установлено, что манифестный гипотиреоз ассоциирован с повышенной опасностью развития ГСД (отношение шансов (ОШ) 1,892; 95% доверительный интервал (ДИ) 1,679–2,132; p <0,001) [24]. Относительный риск развития ГСД также был повышен при субклиническом гипотиреозе, причем ОШ составил 1,558 (95% ДИ 1,292–1,877; p <0,001). В то же время достоверной связи между изолированной гипотироксинемией и вероятностью развития ГСД обнаружено не было (ОШ 1,394; 95% ДИ 0,753–2,580; p=0,291).
Помимо изучения связи недостаточности тиреоидных гормонов, в том числе в исходе аутоиммунного тиреоидита, с развитием ГСД вызывает интерес роль самих антител к структурам щитовидной железы в развитии этого нарушения углеводного обмена. В крупном метаанализе Yang Y. et al., в который вошли результаты 10 когортных исследований и 10 исследований модели «случай–контроль» (n=34 000 пациентов), были получены противоречивые данные [25]. Определение типов антител варьировалось в разных исследованиях. Так, в 9 исследованиях из 20 оценивалось только носительство антител к тиреопероксидазе (АТ-ТПО) [26–34], еще в 10 проводилось определение как АТ-ТПО, так и антител к тиреоглобулину (АТ-ТГ) [35–44], при этом положительным считался результат при носительстве одного из видов антител. В одной статье также были предоставлены результаты по двум видам антител (АТ-ТПО и АТ-ТГ), однако результаты учитывались отдельно для каждого вида антител [45]. В ряде исследований была выявлена ассоциация между носительством антител к щитовидной железе и ГСД, в то время как метаанализ исследований беременных женщин с положительным титром антител к щитовидной железе в I триместре показал отсутствие явного риска развития ГСД по сравнению с контрольной группой. В метаанализах подгрупп не было обнаружено значимой ассоциации между носительством антител к щитовидной железе и ГСД у беременных с эутиреозом, тогда как у женщин с дисфункцией щитовидной железы была выявлена значимая положительная ассоциация [26, 30, 32–34, 38, 40, 41, 44].
Jia M. et al. [46] при исследовании ГСД и субклинического гипотиреоза (СГТ) разделили пациентов на четыре группы в зависимости от триместра и функционального состояния щитовидной железы. Субклинический гипотиреоз в I, II или III триместрах не имел явной связи с риском развития ГСД по сравнению с эутиреоидными женщинами. Однако анализ структуры беременных с СГТ продемонстрировал, что развитие СГТ на ранних сроках беременности было связано с повышенной вероятностью ГСД по сравнению с поздним выявлением СГТ. Также было отмечено, что наличие антитиреоидных аутоантител на ранних сроках гестации приводило к заметному увеличению риска развития ГСД (объединенный ОШ 4,44; 95% ДИ 2,96–6,65; I2=0%).
Biondi B. et al. показали [47], что наличие аутоантител к щитовидной железе не увеличивает риск ГСД у эутиреоидных беременных женщин, их положительный титр в I триместре не имеет прогностической ценности для ГСД. И, наоборот, сочетание высокого уровня ТТГ в сыворотке с аутоантителами к щитовидной железе на ранних сроках беременности было ассоциировано с 4-кратным повышением риска ГСД (ОШ, 4,3; 95% ДИ 2,1–8,9). В ретропроспективном исследовании 6031 беременной женщины в Китае показано, что низкие уровни св. T4 во время беременности были фактором риска ГСД и преэклампсии. И, напротив, частота ГСД снизилась у женщин с более высоким уровнем св. T4, что свидетельствует о защитном эффекте тиреоидных гормонов от развития ГСД [48, 49].
В одной из работ, включавшей 6 когортных исследований, продемонстрировано, что частота ГСД у пациентов с субклиническим гипотиреозом была в 1,35 раза выше, чем в контрольной группе. Риск ГСД увеличивался даже у женщин с высоким уровнем ТТГ в пределах референсных значений [18]. Другой метаанализ [25] показал, что присутствие аутоантител к щитовидной железе не увеличивает вероятность ГСД у эутиреоидных беременных женщин, а их положительность в I триместре не имеет прогностической ценности для ГСД. А вот сочетание высокого уровня ТТГ в сыворотке и аутоиммунитета щитовидной железы на ранних сроках беременности было связано с 4-кратным повышением угрозы ГСД (ОШ 4,3; 95% ДИ 2,1–8,9) и 3-кратным увеличением риска рождения детей с низкой массой тела (ОШ 3,1) [38].
Учитывая высокую распространенность патологии щитовидной железы и нарушений углеводного обмена во время беременности, отсутствие в настоящее время точной информации о взаимосвязи этих патологий, наблюдение за данной группой пациентов и поиск возможных связей остаются актуальными. Ввиду этого на базе кафедры эндокринологии РНИМУ им. Н.И. Пирогова был проведен анализ состояния щитовидной железы у беременных с ГСД.
Цель исследования – изучить особенности функционального состояния щитовидной железы у беременных женщин с ГСД и оценить распространенность гипотиреоза в этой группе пациенток.
МАТЕРИАЛ И МЕТОДЫ
 Исследование проводилось на базе женских консультаций ГКБ им. В.П. Демихова. Был осуществлен ретроспективный анализ амбулаторных карт 779 беременных в возрасте от 19 до 46 лет за 2019 г. Всем женщинам при первичном обращении выполнялось исследование глюкозы плазмы крови натощак, показателей тиреотропного гормона (ТТГ), свободного трийодтиронина (св. Т3), свободного тироксина (св. Т4), АТ ТПО. В случае нормального уровня глюкозы крови при первичном обращении для диагностики ГСД проводился оральный глюкозотолерантный тест.
Исследование проводилось на базе женских консультаций ГКБ им. В.П. Демихова. Был осуществлен ретроспективный анализ амбулаторных карт 779 беременных в возрасте от 19 до 46 лет за 2019 г. Всем женщинам при первичном обращении выполнялось исследование глюкозы плазмы крови натощак, показателей тиреотропного гормона (ТТГ), свободного трийодтиронина (св. Т3), свободного тироксина (св. Т4), АТ ТПО. В случае нормального уровня глюкозы крови при первичном обращении для диагностики ГСД проводился оральный глюкозотолерантный тест.
РЕЗУЛЬТАТЫ
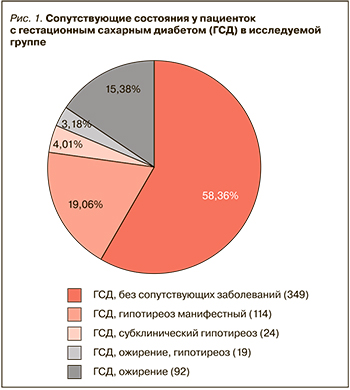
У 599 пациенток был диагностирован ГСД: при этом 349 (58,26%) из них не имели какой-либо сопутствующей патологии, у 114 (19,03%) был выявлен манифестный гипотиреоз (рис. 1). У 92 (15,35%) беременных ГСД развился на фоне ожирения (ИМТ >30 кг/м2), у 19 (3,17%) пациенток с ГСД наблюдалось сочетание ожирения с гипотиреозом. В 19 (3,17%) случаях у женщин с ГСД субклинический гипотиреоз развился в исходе аутоиммунного тиреоидита, т.е. был выявлен положительный титр антител. У 46 беременных женщин было выявлено носительство АТ ТПО при нормальном уровне ТТГ. У 177 беременных женщин с повышенным уровнем ТТГ диагноз ГСД не был подтвержден.
Средний возраст беременных составил 33 года. Чаще всего ГСД выявлялся во II триместре. 74% беременных с ГСД имели ИМТ от 25 до 30 кг/м2, среднее значение 27,3 кг/м2. Средние значения гормональных показателей в исследуемой группе составили: св. Т3 – 3,44 (0,36–15,8) пмоль/л, св. Т4 – 2,33 (0,099–19,4) пмоль/л, ТТГ – 3,15 (0,02–8,72) мМЕ/л (рис. 2). Уровень глюкозы венозной плазмы натощак, по данным проанализированных карт, колебался от 3,46 до 10,9 ммоль/л.
ОБСУЖДЕНИЕ
Из данных проведенного анализа следует, что ГСД чаще возникает у женщин старше 30 лет с избыточной массой тела на момент гестации. ГСД у этой группы беременных часто сочетается с гипотиреозом и носительством АТ ТПО. При наличии этих состояний пациентки имели значительно больший риск развития ГСД по сравнению с эутиреоидными женщинами.
Гипотиреоз, по-видимому, отрицательно сказывается на метаболизме глюкозы, преимущественно влияя на усугубление резистентности к инсулину, но не отражаясь при этом на секреции инсулина поджелудочной железой. Носительство антител к щитовидной железе может быть ассоциировано с увеличением провоспалительных цитокинов, индуцирующих снижение чувствительности к инсулину. Тем не менее механизмы, объясняющие ассоциацию между наличием антител к щитовидной железе и риском ГСД, остаются неуточненными, так как многочисленные эпидемиологические исследования демонстрируют противоречивые результаты.
ЗАКЛЮЧЕНИЕ
Требуется дальнейшее исследование взаимосвязи между гипотиреозом и ГСД. Ее выявление может помочь в ранней диагностике и своевременном лечении этих расстройств.


