Сердечно-сосудистые заболевания (ССЗ) являются наиболее частым осложнением хронической болезни почек (ХБП). У пациентов на ранних стадиях ХБП риск развития ССЗ значительно превышает вероятность прогрессирования патологии до терминальной стадии почечной недостаточности. В последние годы данные научных исследований указывают на то, что минерально-костные нарушения при ХБП (МКН-ХБП) тесно связаны с сердечно-сосудистыми событиями и смертностью [1, 2].
В связи этим идентификация ранних маркеров развития МКН-ХБП и понимание точных механизмов прогрессирования почечного и сердечного повреждения представляются высоко актуальными задачами для разработки новых терапевтических стратегий по снижению сердечно-сосудистой смертности в этой группе пациентов [2, 3].
В настоящее время уровни FGF-23 и белка Клото в сыворотке, которые изначально изучались только в контексте нарушений кальций-фосфорного обмена, считаются одними из ведущих факторов патогенеза ССЗ при ХБП [3–5]. FGF-23 продуцируется остеоцитами и участвует в регуляции метаболизма фосфора, витамина D и паратиреоидного гормона (ПТГ). Белок Клото – необходимый кофактор FGF-23, участвующий в реализации фосфатурического эффекта FGF-23. Также он регулирует выведение кальция почками независимо от FGF-23 [3]. Было установлено, что действие этих факторов при ХБП выходит далеко за рамки только фосфорно-кальциевых нарушений; в последние годы они рассматриваются как важные маркеры сердечно-почечного взаимодействия, которые в значительной степени определяют сердечно-сосудистый риск при ХБП. Высокие уровни FGF-23 в сыворотке (в ответ на задержку фосфатов и снижение экспрессии белка Клото в почечных канальцах при нефросклерозе) связаны с более высоким риском сердечно-сосудистой смертности [3–5]. На сегодняшний день FGF-23 рассматривается как уремический токсин в большей степени из-за его выраженного неблагоприятного воздействия на сердце. Однако точные механизмы этого действия окончательно не установлены.
Белок Клото синтезируется в проксимальных канальцах почек и существует в двух основных формах: трансмембранной (корецептор FGF-23) и секретируемой, которая попадает в кровоток и выполняет эндокринные функции, включая кардиопротекцию [7, 8].
Установлено, что дефицит белка Клото вызывает развитие множественных системных проявлений (в рамках синдрома преждевременного старения), неотъемлемой частью которых выступают тяжелые сердечно-сосудистые нарушения. Снижение уровня белка Клото в сыворотке ассоциировано со снижением скорости клубочковой фильтрации (СКФ), прогрессированием артериальной гипертензии (АГ), сосудистой кальцификации и патологическим ремоделированием сердца и кровеносных сосудов [9–11].
В то же время повышение экспрессии белка Клото продемонстрировало в эксперименте кардиопротективный эффект при нарушении функции почек [10].
Несмотря на активные исследования роли факторов FGF-23 и Клото у пациентов с ХБП, точные механизмы их воздействия на сердечно-сосудистую систему до конца не установлены. Кроме того, до сих пор не имеет убедительного объяснения стойкое повышение уровня тропонина у пациентов с ХБП без клинических проявлений ССЗ [12, 13]. С учетом активного вовлечения FGF-23 и Клото в изменения сердечно-сосудистой системы при ХБП мы провели исследование с целью установить или опровергнуть их возможную роль в развитии ремоделирования сердца и сосудов и повышении уровня тропонина в сыворотке крови.
МАТЕРИАЛ И МЕТОДЫ
Из амбулаторного отделения клиники нефрологии Первого МГМУ им. И.М. Сеченова в исследование были отобраны и включены 130 пациентов (66 мужчин и 64 женщины в возрасте от 20 до 65 лет; средний возраст на момент включения – 47,6±8,4 года) с ХБП 2–5D стадий без клинических симптомов ССЗ (т.е. без ишемической болезни сердца 2–4 функциональных классов по CCS, хронической сердечной недостаточности 2–4 функциональных классов по NYHA, миокардита, перикардита, аритмий) и без тяжелой артериальной гипертензии (с показателями артериального давления ниже 160/90 мм рт.ст.).
Основными этиологическими причинами ХБП в отобранной группе пациентов были хронический гломерулонефрит (латентный и гематурический варианты с минимальной протеинурией <1 г/л), поликистоз почек, хронический тубулоинтерстициальный нефрит (лекарственный и дисметаболический типы).
В дополнение к стандартным методам обследования все пациенты прошли тесты на FGF-23 (Human FGF-23 ELISA kit using antibodies to full FGF-23 molecule, Merk Millipore MILLENZFGF-23-32K), белок Клото (Human soluble Klotho with antiKlotho monoclonal antibodies, IBL-Takara 27998-96Well) в соответствии с протоколом производителя.
Исследование уровня кардиоспецифичного человеческого тропонина проводили иммуноферментным методом для количественного определения кардиоспецифичного тропонина I в человеческой сыворотке (sTr-I ELISA, Biomerica, США).
Инструментальное обследование включало:
- электрокардиографию;
- эхокардиографию (определение индекса массы миокарда левого желудочка, фракции выброса, диастолической функции левого желудочка, оценка кальцификации структур сердца по полуколичественной балльной шкале);
- сфигмографию (определение скорости распространения пульсовой волны, индекса аугментации (ригидности) артерий, субэндокардиального кровоснабжения, центрального (аортального) артериального давления) [14];
- измерение артериального давления (АД) на плечевой артерии.
Критерием гипертрофии левого желудочка (ГЛЖ) служил индекс массы миокарда левого желудочка (ИММЛЖ) >95 г/м2 у женщин и >115 г/м2 у мужчин [15].
Геометрия ЛЖ оценивалась с помощью ИММЛЖ и относительной толщины стенки ЛЖ (ОТС), которая рассчитывалась по следующей формуле:
ОТС = (ТМЖПд + ТЗСЛЖд)/КДР, где ТМЖПд – толщина межжелудочковой перегородки в диастолу; ТЗСЛЖд – толщина задней стенки левого желудочка в диастолу; КДР – конечный диастолический размер левого желудочка.
На основании показателей ИММЛЖ и ОТС выделяли следующие варианты ремоделирования ЛЖ.
1. Нормальная геометрия ЛЖ (нормальный ИММЛЖ, ОТС <0,42).
2. Концентрическое ремоделирование ЛЖ (нормальный ИММЛЖ, ОТС >0,42).
3. Эксцентрическая гипертрофия ЛЖ (увеличение ИММЛЖ, ОТС <0,42).
4. Концентрическая гипертрофия ЛЖ (увеличение ИМЛЖ, ОТС >0,42) [15].
Оценка кальцификации структур сердца (ОКСС) выполнялась с помощью эхокардиографии по полуколичественной балльной шкале [2, 16].
Согласно рекомендациям Американской ассоциации кардиологов (AHA) [14] в отношении центрального артериального давления (ЦАД) и рекомендациям Европейского общества кардиологов по артериальной гипертензии (АГ) [15], значения аортального систолического давления более точно указывают на степень нагрузки на ЛЖ и лучше характеризует повреждающее действие высокого АД на органы-мишени (сердце, почки) в сравнении с периферическим АД, измеренным тонометром на плечевой артерии. В связи с этим ЦАД считается более значимым прогностическим фактором сердечно-сосудистых осложнений и исходов, чем периферическое АД [14, 15].
На основе регистрации пульсовой волны и последующей ее компьютерной обработки (контурный анализ) метод SphygmoСor позволяет неинвазивно измерять аортальное АД и скорость пульсовой волны (СПВ), оценивать субэндокардиальное кровоснабжение (СЭК), а также степень жесткости сосудов и их демпфирующую функцию. Полученные с помощью SphygmoCor результаты сопоставимы со значениями АД при катетеризации аорты и артериального кровотока при компьютерной томографии [17].
СПВ измеряли между общей сонной и бедренной артерией для оценки артериальной жесткости [18, 19].
 Исследование было одобрено локальным этическим комитетом (Первый МГМУ им. И.М. Сеченова, № 07-16, 07.10.2016). Все процедуры выполнялись в соответствии с Хельсинкской декларацией.
Исследование было одобрено локальным этическим комитетом (Первый МГМУ им. И.М. Сеченова, № 07-16, 07.10.2016). Все процедуры выполнялись в соответствии с Хельсинкской декларацией.
Исходные характеристики пациентов оценивались стандартными методами описательной статистики: медианы с межквартильным интервалом, средних значений ± стандартного отклонения и частоты n (%). Для анализа характеристик групп пациентов по среднему уровню показателей, представленных в таблице 1, в зависимости от стадии ХБП (по величине СКФ) использовался также тренд-тест. Связь между двумя переменными определялась с помощью корреляционного анализа, включающего вычисление непараметрического коэффициента ранговой корреляции Спирмена. Значимость различия показателей в двух группах оценивалась посредством критерия хи-квадрат (для качественных переменных) и U-критерия Манна–Уитни (для количественных переменных). Количественные показатели представлены в виде медиан и квартилей, качественные – в процентах.
При выполнении многофакторного анализа использовался метод логистической регрессии. Анализ был скорректирован с учетом влияния дополнительных факторов, которые включали возраст, пол, уровень гемоглобина в сыворотке крови, расчетную СКФ (рСКФ).
Различия считались статистически значимыми при p <0,05. Статистический анализ был выполнен с помощью программы SPSS, версии 21.0 (Чикаго, Иллинойс, США).
РЕЗУЛЬТАТЫ
В таблицах 1 и 2 представлены клинические и лабораторные параметры пациентов, участвовавших в исследовании. Группы пациентов формировались в зависимости от стадии ХБП (величины рСКФ). Между группами не было значительных различий по полу, возрасту, диастолическому АД, индексу массы тела (ИМТ), уровням кальция и альбумина в сыворотке крови.
Уровни центрального систолического и центрального диастолического АД, сывороточного фосфора, ПТГ, FGF-23 и тропонина, а также индекса аугментации и ИММЛЖ прогрессивно увеличивались по мере снижения рСКФ. Уровни гемоглобина и белка Клото уменьшались по мере снижения рСКФ.
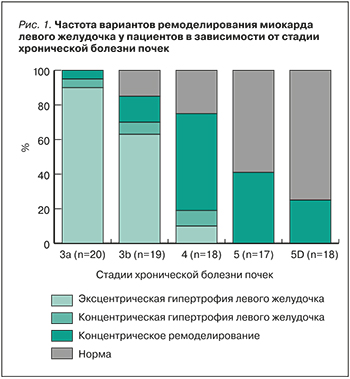
Динамика вариантов ремоделирования миокарда в зависимости от стадии ХБП (3A–5D) представлена на рисунке 1. По мере прогрессирования стадии ХБП доля эксцентрического ремоделирования миокарда увеличивается.
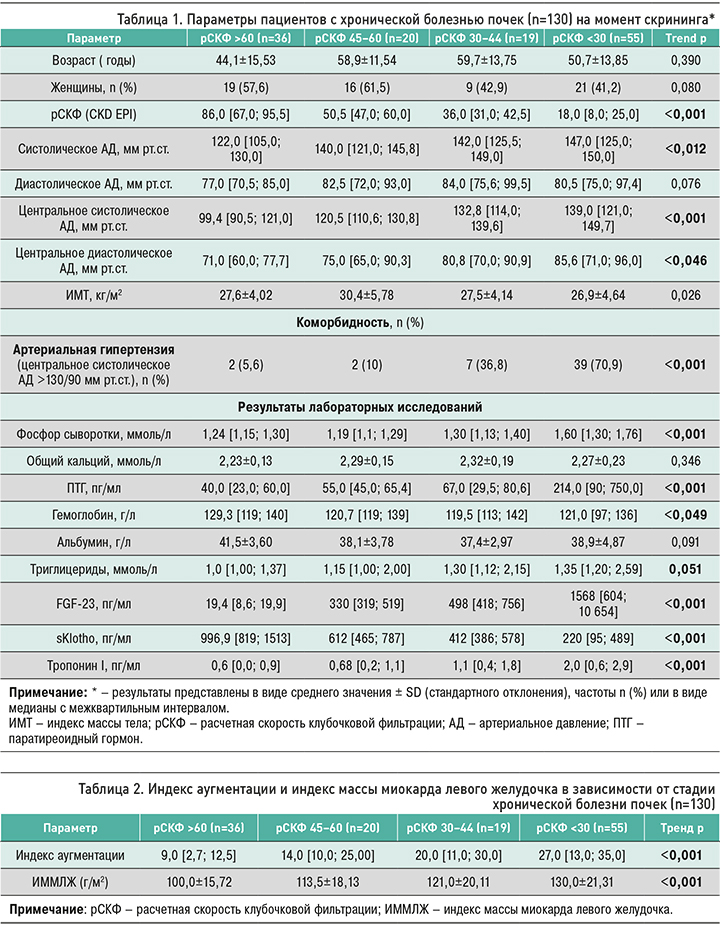
При оценке связи FGF-23 и Клото с вариантами ремоделирования сердца установлена прямая взаимосвязь избыточного уровня FGF-23 преимущественно с эксцентрическим вариантом ремоделирования и обратная между сниженным уровнем Клото и концентрическим вариантом ремоделирования (рис. 2).
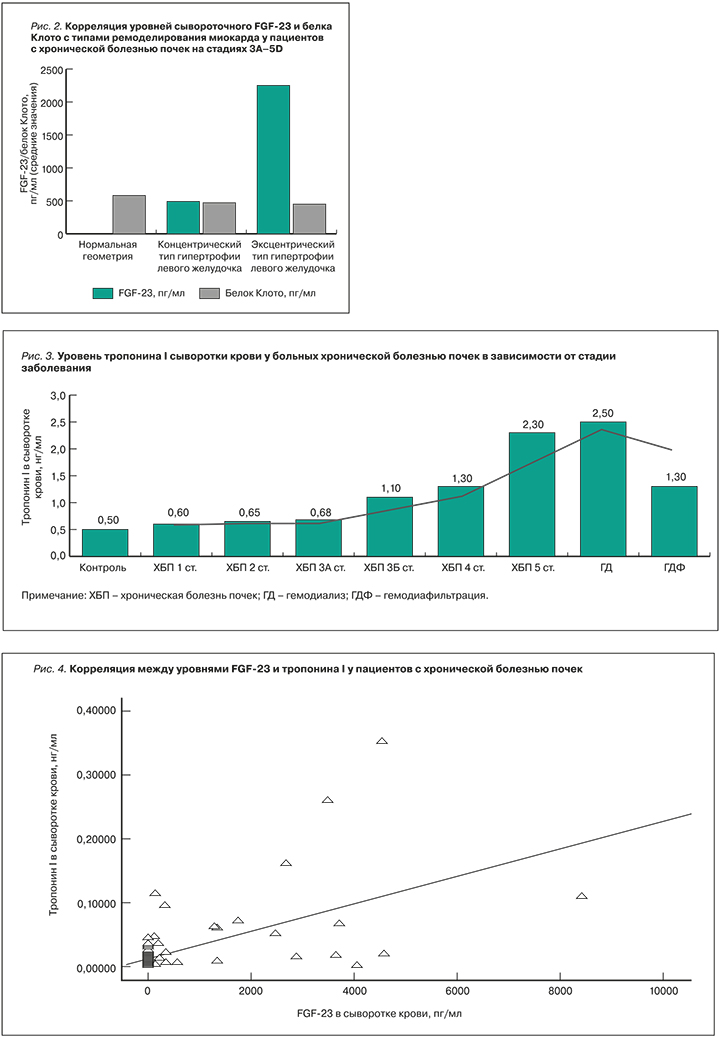
Для уточнения механизмов повреждения кардиомиоцитов нами изучена связь FGF-23, Клото с сывороточным уровнем тропонина I (sTr-I). Подтверждено умеренное повышение сывороточного уровня sTr-I по мере нарастания стадии ХБП у обследованных пациентов без клинической манифестации кардиоваскулярных заболеваний (рис. 3).
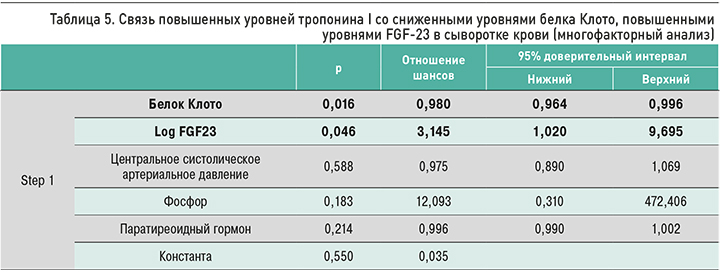
Также нами обнаружена корреляция уровня сывороточного FGF-23 с уровнем sTr-I (r=0,512; p <0,01 – рис. 4), ИММЛЖ (r=0,628; p <0,001), эксцентрической ГЛЖ (r=0,545; p <0,01), диастолической дисфункцией левого желудочка (r=0,448; p <0,05), показателями СЭК (r=-0,469; p <0,05). Уровень сывороточного белка Клото был ассоциирован с более высоким ИММЛЖ (r=-0,489; p <0,01), концентрической ГЛЖ (r =-0,463; p <0,01), диастолической дисфункцией левого желудочка (r = -0,612; p <0,05), повышенной СПВ (r=-0,667; p <0,001), повышенными показателями ОКСС (r=-0,581; p <0,01).
Кроме того, мы выявили различия в сывороточных уровнях FGF-23 и Клото и показателями СЭК (по данным сфигмографии) у больных ХБП (рис. 5).

У пациентов с повышенным (>120/80 мм рт. ст.) и нормальным уровнем ЦАД (90–120/70–79 мм рт. ст.) средние уровни FGF-23 статистически значимо не различались (612+110 и 587+85 соответственно; р=0,071). Это указывает на независимое от уровня ЦАД влияние FGF-23 на миокард.
В то же время уровень sTr-I коррелировал с ИММЛЖ (r=0,590; p <0,05), эксцентрической ГЛЖ (r=0,454; p <0,01), показателями СЭК (r=- 0,449; p <0,01), диастолической дисфункцией левого желудочка (r=0,468; p <0,05), длительностью ХБП (r=0,519; p <0,05), ЦАД (r=0,567; p <0,01).
В свою очередь, уровень белка Клото коррелировал с концентрической ГЛЖ (r=-0,463; p <0,01), диастолической дисфункцией ЛЖ (r=-0,612; p <0,05), повышенной СПВ (r=-0,667; p <0,001), повышенными показателями ОКСС (r=-0,581; p <0,01).
Многофакторный анализ (логистическая регрессия) показал, что эксцентрическая ГЛЖ преимущественно связана с повышенным уровнем сывороточного FGF-23 (отношение шансов (ОШ) =1,056; 95% доверительный интервал (ДИ): 1,006–1,109; p=0,027 – табл. 3), а концентрическая ГЛЖ – со снижением уровня Клото (ОШ=0,988; 95% ДИ: 0,984–0,992; p=0,001 – табл. 4). Также снижение белка Клото было ассоциировано с повышением СПВ (ОШ=0,984; 95% ДИ: 0,977–0,991; p <0,001) и повышенными показателями ОКСС (ОШ=0,991; 95% ДИ: 0,988–0,995; p <0,001) у пациентов с ХБП без манифестации ССЗ.
Кроме того, при многофакторном анализе исследуемых факторов и sTr-I была выявлена связь между уровнями sTr-I и белка Клото, FGF-23 (тaбл. 5).
У пациентов с ХБП 2–5D стадий без клинических проявлений ССЗ повышенные концентрации FGF-23 в сыворотке и пониженные значения сывороточного белка Клото связаны с высоким риском развития кардиоваскулярных заболеваний: уровень FGF-23 – с эксцентрической ГЛЖ (независимо от ЦАД), уровень белка Клото – с концентрической ГЛЖ, повышенными показателями СПВ и ОКСС. Умеренно повышенный уровень sTr-I может быть первым проявлением дисбаланса факторов FGF-23/белка Клото при ХБП.
ОБСУЖДЕНИЕ
Значительный риск ССЗ и смертности у пациентов с ХБП обусловливает острую необходимость поиска надежных биомаркеров, которые позволяли бы своевременно выявлять не только заболевание почек, но и пациентов с более высоким риском сердечно-сосудистой смертности.
По сравнению со «старыми» и уже установленными в клинической практике биомаркерами МКН-ХБП (фосфатами, ПТГ, витамином D), повышенные уровни в крови которых, к сожалению, свидетельствует лишь о продвинутых стадиях ХБП, исследование новых биомаркеров (FGF23, белок Kлото) является многообещающим, поскольку изменение их сывороточного уровня обнаруживается уже на ранних стадиях ХБП и ассоциировано с высоким риском сердечно-сосудистых осложнений [2, 3, 5, 8].
Повышение FGF-23 служит ранним предиктором ССЗ при ХБП и ассоциировано с высокой смертностью [4]. На сегодняшний день FGF-23 рассматривается в качестве нового уремического токсина, чьи уровни в сыворотке возрастают раньше, чем ПТГ, однако точные механизмы влияния FGF-23 при ХБП еще до конца не установлены.
В большом проспективном когортном исследовании Faul C. et al. [21] с участием 3939 больных ХБП было продемонстрировано, что FGF-23 независимо связан с ГЛЖ, развитие которой эксперты KDIGO считают важным патогенетическим механизмом ССЗ у пациентов с ХБП. Лечение блокаторами FGF-23 подавляло прогрессирование ГЛЖ [22].
В то же время результаты наблюдательных исследований, согласно которым выявлена связь между FGF-23 и кальцификацией артерий, вносящей существенный вклад в сердечно-сосудистое повреждение при ХБП, остаются противоречивыми. Ни в одном крупномаштабном исследовании не было выявлено независимой ассоциации уровня FGF-23 с кальцификацией артерий у пациентов с ХБП 2–4 стадий [23]. Наше исследование также не обнаружило статистически значимой связи между FGF-23 и кальцификацией сердца и сосудов (р >0,05). Это дает основание полагать, что непосредственное влияние FGF-23 на ремоделирование сердца более вероятно, чем его влияние FGF-23 на артериальные сосуды, и может лежать в основе ассоциации FGF-23 со смертностью от сердечно-сосудистых причин.
В проспективном когортном исследовании Scialla J.J. et al. [23] с участием 3860 пациентов с 2–4 стадиями ХБП была показана независимая связь повышенного уровня FGF-23 с высоким риском сердечно-сосудистых событий, в особенности среди пациентов с хронической сердечной недостаточностью (ХСН). В своем исследовании авторы установили, что повышенный уровень FGF-23 был связан с более высоким риском ХСН, а не с проявлениями сосудистого атеросклероза. Эти данные указывают на вероятное участие FGF-23 в развитии ХСН, что могло бы объяснить одну из возможных причин высокого риска ССЗ у больных ХБП.
В то же время экспериментальные данные указывают на возможность неселективного связывания избыточного FGF-23 с рецепторами FGF-2 и FGF-4 в сердце, которые участвуют в развитии ГЛЖ и апоптозе кардиомиоцитов [6, 24], что, в свою очередь, также может объяснять влияние FGF-23 на сердце при ХБП.
Наш многофакторный анализ подтверждает гипотезу о наличии связи между FGF-23 и эксцентрической ГЛЖ, на что может указывать бессимптомное повышение уровня sTr-I в сыворотке крови.
Согласно литературным данным, повышенный уровень тропонина у пациентов без клинических проявлений ССЗ, а также в общей популяции, связан с повышением общей смертности и смертности от сердечно-сосудистых причин [25]. Jacobs L.H. et al. обнаружили, что более чувствительный сывороточный тропонин-Т продемонстрировал увеличение более 99-й процентили у 94% пациентов с ХБП [26].
Данные, полученные за последнее десятилетие, позволяют предположить, что повышенный уровень тропонина-Т в сыворотке может предсказать смертность среди пациентов с терминальной почечной недостаточностью без острого коронарного синдрома [22]. Stacy S.R. et al. продемонстрировали, что повышенный уровень тропонина в сыворотке дает возможность идентифицировать лиц с плохим прогнозом среди пациентов с ХБП [27]. Bansal N. et al. показали, что уровень sTr-I в сыворотке тесно связан с сердечной недостаточностью в представленной когорте из 3483 человек с легкой и тяжелой ХБП [28].
Согласно нашим результатам (многофакторный анализ), FGF-23 был независимо ассоциирован с эксцентрической ГЛЖ, что, в свою очередь, можно рассматривать как механизм повышения сердечно-сосудистого риска и смертности от ССЗ.
Повышение уровня FGF23 в сыворотке при ХБП тесно связано со снижением Клото. Установлено, что пациенты с ХБП страдают дефицитом этого белка, который, по имеющимся данным, способствует высокой смертности от ССЗ. Уменьшение уровня Клото при ХБП обнаруживается, начиная со 2 стадии, а в мочевых тестах – уже с 1 стадии [29].
Было показано, что на клеточном уровне циркулирующая форма белка Клото обладает кардиопротективным эффектом, подавляя каналы TRPC6 в сердце в качестве антагониста пути Wnt/b-катенина, который связан с процессом сосудистой кальцификации [30]. У Клото-дефицитных мышей с ХБП отмечались более выраженные ГЛЖ и кальциноз сосудов, чем у мышей дикого типа с ХБП.
Установлено, что эндогенный белок Клото экспрессируется как кардиомиоцитами человека, так и сердечными фибробластами, тогда как уремия или TGF-в1 подавляют его экспрессию в кардиомиоцитах [31]. Повышенная экспрессия белка Клото ингибирует фиброз, индуцированный TGF-в1, и патогенную передачу сигналов Wnt/b-катенина в кардиомиоцитах [31].
Клинические исследования подтверждают экспериментальные данные о кардиопротективной роли белка Клото. У пациентов с ХБП 3 стадии изменения соотношения FGF23/белка Клото коррелировали с изменениями массы левого желудочка [32]. У пациентов на гемодиализе низкие уровни белка Клото были ассоциированы с сердечно-сосудистыми событиями независимо от других факторов МКН-ХБП [33]. Согласно исследованию KNOW-CKD, сывороточный белок Клото выступает независимым биомаркером индекса массы левого желудочка у пациентов с ХБП [34].
По нашим данным, уровень сывороточного белка Клото был независимо связан с параметрами жесткости сосудистой стенки (СПВ), кальцификацией сердечных клапанов и концентрической ГЛЖ. Кроме того, многофакторный анализ исследуемых факторов (Клото, FGF-23, фосфора, ПТГ, центрального систолического АД) и тропонина-I выявил связь уровней тропонина-I с уровнями белка Клото и FGF-23 в сыворотке.
ЗАКЛЮЧЕНИЕ
Результаты исследования и литературные данные позволяют рассматривать FGF-23 и белок Клото в качестве высокоспецифичных маркеров сердечно-сосудистого риска. Они могут быть использованы как одна из терапевтических опций для разработки новых стратегий лечения, направленных на снижение смертности от сердечно-сосудистых причин, продление додиализного периода, а также с целью улучшения качества жизни и снижения затрат на лечение и госпитализацию пациентов с ХБП.


