ВВЕДЕНИЕ
Long-COVID, или постковидный синдром, – общий термин, использующийся для обозначения группы персистирующих симптомов со стороны различных органов и систем, которые наблюдаются у реконвалесцентов острого COVID-19. В конце 2020 г. в связи с растущей актуальностью и высокой распространенностью постковидный синдром был внесен в Международный классификатор болезней 10-го пересмотра (МКБ-10) под кодом U09.9 – Состояние после COVID-19 [1].
Результаты систематического анализа 25 наблюдательных исследований, проведенного в 2021 г., сообщают о наличии хотя бы одного симптома Long-COVID у 80% пациентов [2]. Согласно National Institute for Health and Care Excellence (NICE), длительность симптомов, связанных исключительно с перенесенным COVID-19 и не имеющих объяснения в контексте других диагнозов, может составлять более 4 нед [3]. Данные зарубежного наблюдательного исследования свидетельствуют о сохранении постковидных симптомов у 77,1% пациентов даже спустя 12 мес после COVID-19 [2]. К факторам риска развития длительного постковидного синдрома относятся тяжелое течение COVID-19, пожилой возраст, принадлежность к женскому полу, наличие сопутствующих заболеваний [2]. Тем не менее признаки Long-COVID нередко наблюдаются и у пациентов с легким и даже бессимптомным течением острой инфекции [4].
МЕХАНИЗМЫ ПОРАЖЕНИЯ РАЗЛИЧНЫХ ОРГАНОВ И СИСТЕМ ПРИ COVID-19 И LONG-COVID
Разнообразие клинических проявлений острого СOVID-19 и Long-COVID связано с мультисистемностью поражений, индуцируемых SARS-CoV-2. Нарушение функции дыхательной системы происходит вследствие связывания вируса с рецепторами ангиотензинпревращающего фермента 2 (АПФ 2) на поверхности альвеолоцитов при участии трансмембранной сериновой протеазы 2 (TMPRSS2) с индукцией воспалительного каскада, развитием диффузного альвеолярного повреждения и гипоксии [5]. Все это приводит к классическим для COVID-19 клиническим признакам – одышке, снижению сатурации, дыхательной недостаточности, острому респираторному дистресс-синдрому (ОРДС).
Поражение нервной системы происходит в результате проникновения SARS-CoV-2 в центральную нервную систему (ЦНС) по обонятельным путям или через гематоэнцефалический барьер, функция которого нарушается из-за влияния вируса на эндотелиальные клетки [6]. Неврологические симптомы COVID-19 и, как следствие, постковидного синдрома обусловлены действием SARS-CoV-2 на рецепторы АПФ 2 в ЦНС, нейровоспалением, эксайтотоксичностью, гипоксией и гипоксемией (из-за поражения легких и нарушения газообмена), микротромбообразованием и ухудшением кровоснабжения нервной ткани [7–9]. Согласно данным ПЭТ в исследованиях Delorme C. et al. и Guedj E. et al., при COVID-19 наблюдается снижение церебрального метаболизма, в частности гипометаболизм глюкозы; это может отражаться на интенсивности протекания энергетических процессов в нейронах [9]. Высокий уровень провоспалительных цитокинов в ткани головного мозга (как часть нейровоспалительного процесса) и гипоксия при COVID-19 способствуют снижению синтеза серотонина, превращая его предшественник, триптофан, в кинуренин, метаболизм которого приводит к снижению выброса дофамина [10]. В результате сочетанного действия всех факторов повреждения нервной системы вследствие инфицирования SARS-CoV-2 проявления COVID-19 и Long-COVID включают персистирующие эмоциональные нарушения, астению и когнитивные нарушения (постковидный неврологический синдром).
Обилие рецепторов АПФ 2 и TMPRSS2 в эпителии желудочно-кишечного тракта (ЖКТ) делает его уязвимым для проникновения SARS-CoV-2 [11]. Механизм повреждения ЖКТ при COVID-19 обусловлен прямым цитотоксическим действием вируса на кишечный эпителий, воспалительной реакцией на поражение эпителия, микротромбообразованием, нарушением регуляции ренин-ангиотензин-альдостероновой системы и мальабсорбцией триптофана – ключевой незаменимой аминокислоты, играющей важную роль в поддержании гомеостаза кишечника и регуляции экспрессии антимикробных пептидов, которые влияют на состав микробиоты [12]. Результаты многоцентрового наблюдательного исследования композиции микробиома толстой кишки группы пациентов с верифицированной COVID-19 инфекцией и группы контроля с применением метода секвенирования ДНК показали статистически значимое различие в составе микробиоты между группами. Продемонстрированное в этом исследовании сохранение дисбиоза после разрешения острого периода заболевания может способствовать персистенции симптомов со стороны ЖКТ в рамках Long-COVID [13]. Имеются также данные о том, что в 36% случаев после острых инфекционных поражений ЖКТ развивается постинфекционный синдром раздраженного кишечника (СРК) [14].
В последнее время появляются данные о возможной роли синдрома активации тучных клеток в патогенезе воспалительных изменений, индуцируемых SARS-CoV-2. В пользу этого говорит схожесть проявлений COVID-19 и Long-COVID с симптомами, наблюдаемыми при синдроме активации тучных клеток, а также результаты эпидемиологических исследований, в которых показано положительное влияние систематического приема блокаторов Н1-гистаминовых рецепторов 2-го поколения на устойчивость к инфекции SARS- CoV-2 и на тяжесть течения заболевания вследствие влияния на уровень воспаления [15, 16].
ЗАРУБЕЖНЫЕ ДАННЫЕ О РАСПРОСТРАНЕННОСТИ СИМПТОМОВ LONG-COVID
Первые международные исследования по оценке распространенности постковидных симптомов, выполненные в 2020 г., подтвердили предположения о наличии у реконвалесцентов острого COVID-19 остаточных признаков повреждения не только респираторной системы в виде кашля и одышки, но и нервной, пищеварительной и других систем в результате прямого или опосредованного влияния SARS-CoV-2 [17–19].
В систематическом анализе 39 исследований в 2021 г. получены следующие результаты по частоте встречаемости различных симптомов Long-COVID: астения присутствовала у 44% пациентов, одышка – у 40%, тревога – у 34%, нарушения сна – у 33%, сниженное настроение – у 32%, кашель – у 22% [20]. По данным отдельных исследований, 56,3% реконвалесцентов острого COVID-19 в течение 12 мес сталкиваются с низкой переносимостью физических нагрузок, 50% – с когнитивными нарушениями, 36,9% – с трудностями в концентрации внимания [21, 22]. Более 44% пациентов испытывают головную боль, 22–25% – боль в мышцах и суставах, 17% – диарею, 7,5% – дискомфорт/боль в животе [17, 23–26].
В целом неврологические, пульмонологические и гастроэнтерологические симптомы лидируют среди всех симптомов Long-COVID. Также имеются сведения о том, что у пациентов с наличием хронических неврологических, пульмонологических и гастроэнтерологических заболеваний в анамнезе выраженность симптомов поражения нервной, дыхательной и пищеварительной систем после перенесенного COVID-19 может существенно увеличиваться [26–28].
Согласно метаанализу Malik P. et al., 58% пациентов, перенесших COVID-19, имеют низкий уровень качества жизни. Причиной тому почти в половине случаев является дискомфорт, связанный с сохраняющимися симптомами, в 37% – развитие тревожного/депрессивного расстройства, когнитивных нарушений, в 28% – низкая переносимость нагрузок, которая отражается на выполнении ежедневных активностей [29].
РЕЗУЛЬТАТЫ ВСЕРОССИЙСКОГО ИССЛЕДОВАНИЯ РАСПРОСТРАНЕННОСТИ СИМПТОМОВ LONG-COVID
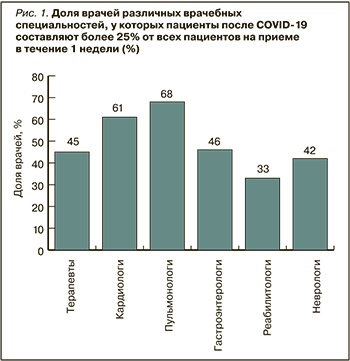
В сентябре 2021 г. Российским научным медицинским обществом терапевтов (РНМОТ) был проведен всероссийский опрос 720 врачей различных специальностей: терапевтов и врачей общей практики (n=533; 74,1%), кардиологов (n=83; 11,5%), пульмонологов (n=47; 6,5%), гастроэнтерологов (n=37; 5%), неврологов (n=17; 2,4%) и реабилитологов (n=3; 0,4%). Доля различных специалистов, у которых пациенты после COVID-19 составляют более 25% от всех пациентов на приеме в течение 1 нед, оказалась равной 30–60%. Чаще всего реконвалесценты острого COVID-19 обращаются к пульмонологам, кардиологам и терапевтам (рис. 1). На приеме у 25% терапевтов и кардиологов, 30% неврологов, 32% гастроэнтерологов, 47% пульмонологов больше 50% всех посещений в неделю приходится на пациентов после перенесенного COVID-19.
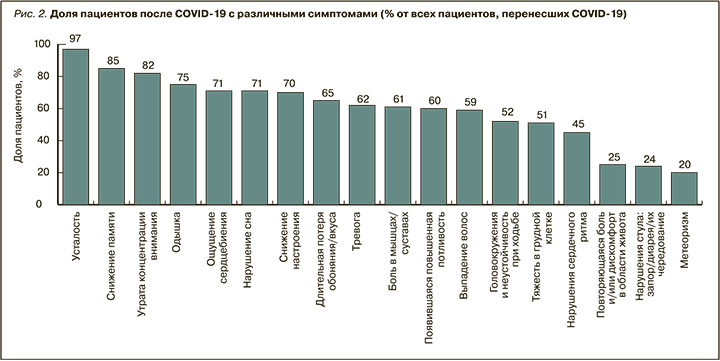
Распределение симптомов, выявляемых у пациентов в постковидном периоде на приеме у врачей всех опрошенных специальностей по всем регионам России, представлено на рисунке 2. Наиболее частыми симптомами являются неврологические и респираторные нарушения: усталость/утомляемость (97%), когнитивные нарушения (снижение памяти у 85%, внимания – 82%), одышка (75%). Несколько реже встречаются признаки тревоги и сниженного настроения (62–70%), головокружение (52%) и гастроэнтерологические симптомы – дискомфорт в животе, нарушение стула, метеоризм (20–25%).
Был проведен анализ частоты встречаемости постковидных симптомов среди пациентов на приеме у врачей-терапевтов, неврологов, гастроэнтерологов (рис. 3). Основными среди этих симптомов как на приеме у терапевтов, так и у неврологов выступают усталость и снижение памяти (98 и 87% соответственно в случае с терапевтами, 100 и 97% соответственно в случае неврологов). Поводами для обращения к гастроэнтерологу у пациентов становятся метеоризм (83%), нарушение стула – запор/диарея/их чередование (82%), усталость (79%) и дискомфорт/боль в животе (77%). При посещении пульмонолога пациенты после перенесенного COVID-19 сообщают об усталости (92%), снижении настроения (73%) и одышке (69%).
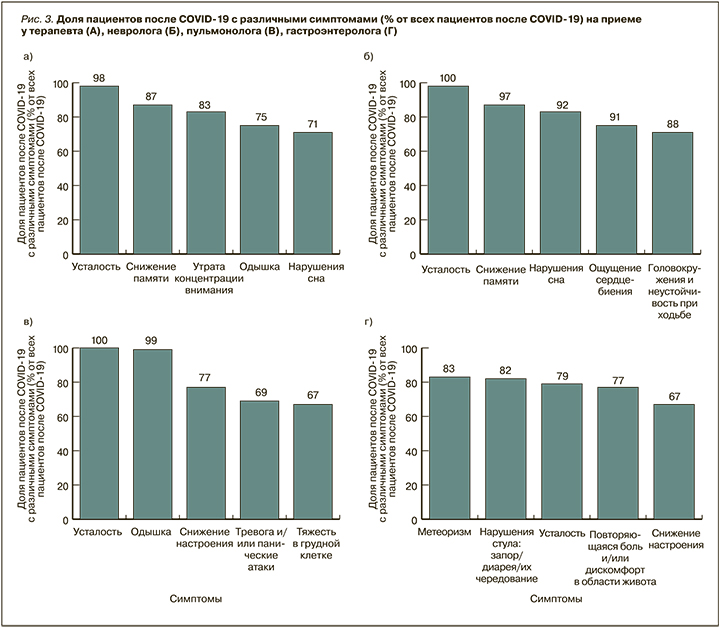
Таким образом, пациенты после перенесенного COVID-19 составляют более четверти пациентов у 33–68% врачей различных специальностей, более половины – у 25–47% врачей. Наиболее часто встречающиеся постковидные симптомы среди пациентов на приеме у любых специалистов в российской клинической практике – неврологические и пульмонологические. На приеме у гастроэнтеролога чаще других симптомов встречаются нарушение стула и дискомфорт в животе.
ТЕРАПИЯ СИМПТОМОВ LONG-COVID
Согласно данным зарубежной литературы, рекомендуемыми методами терапии пациентов с Long-COVID служат респираторная реабилитация, умеренная физическая нагрузка и психотерапия [30–32]. Ведутся отдельные исследования по использованию антидепрессантов, ноотропов, антиоксидантов, пробиотиков, противовоспалительных средств [33]. Тем не менее единый алгоритм реабилитации постковидных пациентов еще не принят.
В российской практике в реабилитации пациентов с Long-COVID используются Временные методические рекомендации (ВМР) «Медицинская реабилитация при новой коронавирусной инфекции COVID-19» (версия 2, 31.07.2020) [34], а также Методические рекомендации РНМОТ «Медицинская реабилитация больных, перенесших COVID-19 инфекцию» [35].
На амбулаторном этапе ведения реконвалесцентов острого COVID-19 предлагаются комплекс респираторной реабилитации (дыхательная гимнастика, CPAP, физиотерапия, ингаляции муколитиков), поддержание физической активности, массаж. Для лечения эмоциональных нарушений рекомендована психотерапия [34]. Медикаментозные способы терапии эмоциональных нарушений, несмотря на их эффективность, в ВМР не описаны. К сожалению, в ВМР также не представлены способы помощи пациентам с когнитивными нарушениями, астенией, головокружением, нарушением пищеварительной функции, в то время как результаты зарубежных и российских исследований (опрос, проведенный РНМОТ) указывают на большую распространенность этих симптомов и их влияние на качество жизни. В настоящее время совместными усилиями РНМОТ, Национального научного общества инфекционистов и Союза реабилитологов России подготовлены к печати и одобрены методические рекомендации «Особенности течения Long-COVID-инфекции, терапевтические и реабилитационные мероприятия», в которых уже представлены подробные сведения о возможностях ее медикаментозной коррекции.
Разработка алгоритмов комплексной (как немедикаментозной, так и медикаментозной) терапии разнообразных постковидных симптомов по-прежнему остается актуальной. В России проводятся исследования по использованию средств фармакотерапии для патогенетического лечения отдельных симптомов Long-COVID, однако они еще далеки от завершения. При этом количество пациентов с выраженными постковидными нарушениями и связанными с ними низкими показателями качества жизни только увеличивается. В сложившейся ситуации целесообразно рассмотреть применение препаратов, доказавших свою эффективность и безопасность в терапии отдельных симптомов (астении, одышки, нарушений пищеварения), а также продолжительного воспалительного синдрома еще до начала пандемии COVID-19.
В последнее время получены свидетельства того, что блокаторы H1-гистаминовых рецепторов 2-го поколения, наряду с некоторым другими препаратами, оказывают положительное влияние на выраженность симптомов и на смертность пациентов с новой коронавирусной инфекцией [16, 36]. С учетом этих доказательств, а также явной взаимосвязи патогенеза COVID-19 и синдрома активации тучных клеток была высказана гипотеза о возможности использования этих препаратов в том числе в постковидном периоде [16, 36–40].
Один из новых антигистаминных препаратов 2-го поколения – Теоретин® (МНН – бензгидрилпиперазинилбутилметилксантина сукцинат). Препарат был получен с помощью модификации метилксантиновых производных фармакоформным фрагментом современного высокоэффективного блокатора Н1-рецепторов цетиризина. Согласно полученным данным, Теоритин® обладает выраженной антигистаминной и противовоспалительной активностью, проявляющейся в достоверном снижении индуцированной продукции ряда провоспалительных цитокинов (интерлейкина-6, интерлейкина-8 и фактора некроза опухоли-альфа) и сравнимой с действием дексаметазона [41]. Имеется убедительный опыт эффективного и безопасного применения Теоритина® в лечении пациентов с аллергическим ринитом и хронической идиопатической крапивницей [42, 43]. Существующие доказательства способности метилксантиновых производных регулировать активность тучных клеток, положительно влиять на мозговой кровоток, защищать нейроны от эксайтотоксичности, а также появившиеся свидетельства актуальности применения этой группы препаратов для лечения COVID-19 дают основания рассматривать Теоритин® как перспективный препарат для применения у пациентов с постковидным симптомокомплексом [44–47].
В лечении когнитивных нарушений и астении хорошо зарекомендовал себя ноотропный препарат Нанотропил® Ново (МНН: фонтурацетам). Он является пирролидоновым производным, способным влиять на причины когнитивных нарушений, увеличивать количество NMDA, дофаминовых и н-ацетилхолиновых рецепторов, участвующих в процессах формирования памяти и внимания, увеличивать содержание дофамина, серотонина, норадреналина в головном мозге, повышать усвоение глюкозы, обеспечивая повышение энергетического потенциала нервных клеток [48, 49]. Влияние фонтурацетама на обратный переносчик дофамина в синапсах позволяет в патологических условиях поддерживать концентрацию дофамина, защищающую нейроны от эксайтотоксичности [50, 51]. В двойном слепом плацебо-контролируемом рандомизированном исследовании препарата у пациентов с когнитивными нарушениями было показано значимое улучшение когнитивных функций по шкале MMSE, снижение астении по шкале MFI-20, повышение внимания и скорости умственных процессов, выражавшееся в увеличении скорости выполнения заданий теста Шульте (р >0,05 по сравнению с плацебо) на фоне хорошей переносимости терапии [52]. В другом сравнительном исследовании с участием пациентов с когнитивными нарушениями препарат приводил к значимому клиническому улучшению у 62% пациентов, положительно влиял на функцию памяти и уменьшал астению [53]. Кроме того, показано положительное влияние фонтурацетама на качество жизни [54]. Нейрометаболическое, нейропротекторное, антиастеническое, адаптогенное, ноотропное действие препарата Нанотропил® Ново может быть использовано в целях купирования нарушений памяти, внимания и усталости, столь часто встречающихся у пациентов на приеме у различных специалистов.
Течение и прогноз развития COVID-19 усугубляются запускаемым вирусом SARS-CoV-2 процессом воспаления и его последствиями: «цитокиновым штормом», повышением концентрации активных форм кислорода и метаболитов, нарушением и депривацией антиоксидантных механизмов с развитием оксидантного стресса, гипоксией, нарушением механизмов свертывания и реологии крови [55, 56]. Для воздействия на эти патогенетические механизмы возможно использование этилметилгидроксипиридина малата (Этоксидол®). Этоксидол® способствует нормализации как метаболических нарушений, так и сопутствующих неврологических расстройств, а также профилактике осложнений эндотелиальной дисфункции, которые распространены у пациентов в постковидном периоде. Этоксидол® является ингибитором свободно-радикальных процессов, оказывает мембранопротекторное, антитоксическое, антиишемическое и антигипоксантное действие, повышает устойчивость организма к стрессу, в том числе у пациентов с коронавирусной инфекцией [4, 56–60].
В клинических исследованиях был подтвержден комплексный механизм действия препарата Этоксидол® и продемонстрирована обоснованность его применения в лечении COVID-19 и других заболеваний [59–61]. Действие препарата Этоксидол® при COVID-19 основано на положительном сдвиге ключевых звеньев патогенеза, что влияет на течение и профилактику осложнений заболевания. Препарат повышает активность ферментов антиоксидантной защиты супероксиддисмутазы и каталазы, ингибирует перекисное окисление липидов, уменьшает содержание общих органических перекисей, редуцирует оксидантный стресс, препятствует накоплению первичных продуктов перекисного окисления липидов (лактата). Доказано, что Этоксидол® способствует энергопродукции в ишемизированной клетке при накоплении недоокисленных продуктов и ацидозе, ограничивает зону ишемического повреждения, улучшает кровоток в зоне ишемии, микроциркуляцию и реологию крови, уменьшает агрегацию тромбоцитов.
Этоксидол® способствует ограничению воспалительного процесса в легких за счет увеличения концентрации фоллистатина [4, 58–61]. В исследовании В.Г. Кукес с соавт. изучалось действие этого препарата на концентрацию метаболитов окислительного стресса, а также динамику парциального давления кислорода в артериальной крови в условиях окислительного стресса [61]. Терапия препаратом Этоксидол® приводила к статистически значимому повышению уровня парциального давления кислорода в артериальной крови, снижению общей концентрации органических перекисей, уменьшению уровня лактата. Полученные данные позволяют заключить, что Этоксидол® улучшает результаты лечения и профилактики ряда состояний за счет повышения активности ферментов антиоксидантной системы и уменьшения выраженности оксидантного стресса [61]. По результатам исследования было показано выраженное действие препарата Этоксидол® в снижении неврологической симптоматики (головокружения, нарушений равновесия, шума в ушах), улучшении общего состояния и повышении качества жизни [29]. Антиоксидант нового поколения Этоксидол® рекомендован экспертами для использования в различные периоды терапии COVID-19 [4, 55–58].
Тералиджен® (МНН: алимемазин) – анксиолитическое и седативное средство. Этот препарат проявляет мультимодальное действие, влияя на различные типы рецепторных систем (α-адренергическую, Н1-гистаминовую, серотониновую, D2-дофаминовую, М-холинергическую). Тералиджен® обладает анксиолитическим, седативным, гипнотическим, вегетостабилизирующим и антигистаминным эффектами, уменьшая возбуждение, тревогу, фобию, беспокойство, агрессию, вегетативные/соматоформные симптомы. Препарат применяется в терапии психоповеденческих расстройств (аффективных тревожно-депрессивных, невротических, генерализованного тревожного, фобического, ипохондрического), вегетативной дисфункции (соматоформных расстройств) и др. Отличительным от большинства психоактивных препаратов свойством Тералиджена® является редкость и незначительная выраженность побочных эффектов, связанных с его приемом. С учетом этих свойств препарат может использоваться на различных этапах лечения пациентов с COVID-19 для коррекции широкого спектра заболеваний с психоэмоциональными, поведенческими, невротическими, вегетативными расстройствами, бессонницей [4, 62–65].
В скрининговом тесте на ингибиторы проникновения вируса SARS-CoV-2 с использованием псевдотипированного вируса SARS2-S среди 1800 низкомолекулярных лекарственных средств тримепразин (алимемазин) оказался многообещающим ингибитором SARS-CoV-2, блокируя стадию проникновения вируса (репликацию нативного вируса в клетках Vero E6) в сравнении с контролем. Алимемазин также проявляет противовоспалительный эффект (уменьшает активность NF-κB через фосфолипазу C и фосфатидилинозитол сигнальные пути, снижает презентацию антигена и экспрессию провоспалительных цитокинов, молекул клеточной адгезии и хемотаксических факторов) [66, 67]. Включение препарата Тералиджен® в терапию пациентов с COVID-19 и психоэмоциональными расстройствами (в комбинации с базовой терапией) вызывало быструю редукцию психовегетативной симптоматики, в том числе в случаях, резистентных к назначению других лекарственных средств [68]. В другом исследовании назначение пациентам с COVID-19 Тералиджена® оказалось эффективным в плане быстрого купирования нозогенных психических реакций, тревожно-фобических и вегетативных симптомов, включая гиперсимпатикотонию, был показан клинически сбалансированный снотворный, седативный и антигистаминный эффект препарата [66].
Антигипоксантный препарат Гипоксен® (МНН: полидигидроксифенилентиосульфонат натрия) обладает способностью шунтировать 1-й и 2-й комплексы дыхательной цепи митохондрий, восстанавливая работу дыхательной цепи в условиях гипоксии, подавлять эндогенное образование свободных радикалов в митохондриях и снижать сродство эритроцита к кислороду путем изменения конформации порфирина и модификации его ионотранспортных систем; все это приводит к более легкому проникновению кислорода, связанного гемоглобином, в клетки [69–72]. Исследования препарата Гипоксен® с участием пациентов с заболеваниями органов дыхания продемонстрировали его эффективность в укорочении сроков выздоровления после острого заболевания, снижение на фоне его приема выраженности одышки (по TDI), повышение переносимости физической нагрузки (по результатам 6-минутного шагового теста), сокращение сроков восстановления кардиореспираторной системы после физической активности, уменьшение выраженности десатурации на фоне нагрузки и увеличение качества жизни у пациентов с хроническими респираторными заболеваниями [70, 71].
Тримебутин (Тримедат®), воздействуя на энкефалинергическую систему ЖКТ, нормализует висцеральную чувствительность и моторику на всем его протяжении вне зависимости от типа нарушения моторики, т.е. оказывает спазмолитическое действие при гиперкинетических состояниях и стимулирующее при гипокинетических [73]. Благодаря этому тримебутин способствует устранению широкого круга симптомов функциональных заболеваний ЖКТ (боли, спазмов и дискомфорта в области живота, метеоризма и вздутия живота, диареи, запора, диспепсии, тошноты и рвоты), что продемонстрировано в клинических исследованиях, в том числе в метаанализе Кокрейновской библиотеки [74–76]. В исследованиях подтверждено положительное влияние тримебутина на качество жизни пациентов [77, 79].
В контексте проблемы нарушения функции ЖКТ, связанной с COVID-19, представляется важной способность тримебутина оказывать противовоспалительное действие, что было показано в статье Ogawa N. еt al. [79]. В метаанализе Poynard T. еt al. (2001) было показано, что разница между тримебутином в терапевтических дозах и плацебо в отношении нежелательных явлений статистически не значима [80]. Комплексный механизм действия тримебутина помогает избежать полипрагмазии и снизить лекарственную нагрузку на пациента.
Вовлечение дисбиотических изменений в кишечнике в формирование постинфекционных функциональных заболеваний ЖКТ делает целесообразным назначение пробиотиков. В плацебо-контролируемом исследовании было показано, что применение пробиотического комплекса, содержащего комбинацию штаммов Bifidobacterium bifidum, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus rhamnosus, сопровождалось уменьшением выраженности симптомов СРК и устранением синдрома избыточного бактериального роста в тонкой кишке [81]. В другом исследовании было показано, что добавление пробиотика к тримебутину повышает эффективность лечения функциональных заболеваний ЖКТ [82].
Таким образом, к настоящему времени доступна эффективная и безопасная терапия с подтвержденной способностью купировать неврологические, пульмонологические и гастроэнтерологические симптомы, персистирующий воспалительный синдром, которая может применяться для симптоматического лечения проявлений Long-COVID с целью улучшения состояния пациентов в постковидном периоде и повышения качества жизни за счет улучшения памяти, концентрации, повышения активности, снижения тревоги и нормализации функции пищеварительной системы.
ЗАКЛЮЧЕНИЕ
Long-COVID представляет собой сложный симптомокомплекс, который характерен для большинства переболевших COVID-19. По результатам как зарубежных исследований, так и данным опроса врачей – членов РНМОТ, наиболее распространенными в этом случае являются неврологические, пульмонологические и гастроэнтерологические симптомы. Среди первых, по результатам проведенного опроса, преобладают усталость, когнитивные нарушения и психоэмоциональные нарушения, среди вторых – одышка, среди третьих – нарушение стула.
С учетом большого влияния разнообразных признаков Long-COVID на качество жизни пациентов необходима разработка подходов к терапии постковидного синдрома. Имеющиеся в настоящее время ВМР не включают реабилитационные мероприятия, направленные на купирование неврологических и гастроэнтерологических симптомов, а исследования возможности использования патогенетической терапии еще не завершены, поэтому применение препаратов с доказанной эффективностью в отношении наиболее распространенных симптомов Long-COVID является обоснованной и целесообразной мерой. К таким препаратам относятся ноотроп Нанотропил® Ново, направленный на снижение выраженности когнитивных симптомов, Тералиджен®, способствующий уменьшению выраженности психоэмоциональных нарушений, антиоксидант Этоксидол® и антигипоксант Гипоксен®, улучшающие процессы тканевого дыхания и защиты от свободно-радикального окисления, препараты Тримедат® и Теоретин® – лекарственные средства, доказавшие свою эффективность и безопасность в клинических исследованиях, том числе в двойных слепых рандомизированных с плацебо-контролем.


