ВВЕДЕНИЕ
По данным экспертов Всемирной организации здравоохранения, дефицит железа (ДЖ) занимает первое место среди 38 наиболее распространенных заболеваний человека: примерно одна треть населения мира (32,9%) страдала от анемии в 2010 г. [1].
Последние исследования убедительно свидетельствуют о важной роли гепсидина в метаболизме железа. Гепсидин регулирует скорость всасывания железа, контролируя экспрессию ферропортина-1 на базолатеральных мембранах энтероцитов. Исходя из этого, предполагается, что высвобождение железа из клеток эпителия крипт кишечника, печени и макрофагов снижается, когда уровни гепсидина повышаются при перегрузке железом или воспалении (через интерлейкин 6). Напротив, вполне вероятно, что экспрессия ферропортина-1 и высвобождение железа повышаются при снижении уровня гепсидина, как это имеет место при ДЖ, железодефицитной анемии (ЖДА) или гипоксии [2]. Исследования на трансгенных мышах с увеличенной продукцией гена USF-2 (гена прогепсидина) убедительно доказали, что повышенная экспрессия гепсидина ведет к дефициту железа [3–6]. Результаты экспериментальных и клинических исследований продемонстрировали, что инфекция и воспаление вызывают гиперпродукцию гепсидина и развитие ДЖ [3–5, 7]. Экспрессия синтеза гепсидина при хронических воспалительных заболеваниях приводит к уменьшению абсорбции железа в кишечнике [8–9]. В этом случае развивается дефицит железа, особенностью которого является снижение содержания железа в сыворотке при нормальном или повышенном его уровне в клетках ретикулоэндотелиальной системы [10].
Продолжает обсуждаться вопрос, насколько повышенный уровень гепсидина будет изменять метаболизм препаратов железа, применяемых для компенсации возникающего ДЖ. Вероятно, гепсидин блокирует транспорт железа в эпителии кишечника и макрофагах [11]. При использовании в исследовании стабильных изотопов железа в составе сульфата железа (60–240 мг) было установлено, что прием железа вызывает увеличение гепсидина на срок до 48 ч, ограничивая абсорбцию последующих доз [12].
Цель исследования – изучить фармакокинетику препаратов железа у пациентов с ДЖ, возникшем на фоне анемии хронических заболеваний (АХЗ), по сравнению с пациентами с изолированной ЖДА.
МАТЕРИАЛ И МЕТОДЫ
Исследование фармакокинетики железа проводилось в течение 2 дней. В первый день отбирали образцы крови с 08:00 до 18:00 через каждый час для определения динамики базальных концентраций железа в сыворотке крови в течение дня. На второй день в 08:00 у больных отбирали первую пробу крови, затем больные принимали 2 таблетки препарата железа, содержащие суммарно 200 мг железа и 120 мг аскорбиновой кислоты. После этого пробы отбирали каждый час до 18:00. В течение двух дней исследования все больные не употребляли чай, кофе, шоколад и получали стандартизованную диету, содержащую 4,8 мг железа. Пробы центрифугировали, и в течение 2 ч после взятия крови в сыворотке крови определяли уровень железа реактивами «Железо» (Iron FZ; фирма СHEMA DIAGNOSTICA, Италия) на автоматическом биохимическом анализаторе («Олимпус XL-640», Германия).
Гепсидин исследовался количественно в сыворотке крови методом твердофазного иммуноферментного анализа по принципу конкурентного связывания при помощи реактивов «Гепсидин-25» (без экстракции; BCM Diagnostics). За нормальный уровень гепсидина принимали значения от 2,3 до 6,5 нг/мл, которые составляли 99% доверительный интервал (ДИ) результатов исследования уровня гепсидина у 30 здоровых добровольцев (15 мужчин, 15 женщин, средний возраст 23,1±2,4 лет).
После того как концентрация железа была определена в образцах, для каждого больного строилась фармакокинетическая кривая, рассчитывалась площадь под кривой (AUC) базального уровня железа и после приема 200 мг сульфата железа. В каждой группе больных определялся коэффициент вариации (CV) AUC. Для расчета итоговой фармакокинетической кривой, показателей Сmax, Tmax во всех определениях были вычтены уровня железа в сыворотке крови до начала приема препарата в 08:00.
В исследование было включено 15 женщин с ЖДА и 15 женщин с ДЖ на фоне АХЗ (АХЗ + ДЖ). Клиническая характеристика участников представлена в таблице 1.
Все пациенты подписали информированное согласие на участие в исследовании, протокол исследования был одобрен локальным этическим комитетом ФГАОУ ВО «Первый Московский государственный медицинский университет им. И.М. Сеченова» Минздрава России (Сеченовский Университет), протокол от 28.04.2021 № 07-21.
Статистическую обработку результатов исследования проводили при помощи программы MedCalc (версия 18.11) для Windows XP Vista. Для определения нормальности распределения в группах использовали критерий Колмогорова–Смирнова, при p <0,05 гипотеза нормальности распределения отвергалась. В случае нормального распределения значения представлялись в виде средней (M) и среднего квадратичного отклонения (σ). Для установления разницы между группами использовались критерии Стьюдента (t), при сравнении данных до и после воздействия применялся парный критерий Стьюдента (t). Значения считали статистически значимыми при p <0,05.
РЕЗУЛЬТАТЫ И ОБСУЖДЕНИЕ
Среди пациенток, включенных в исследование, не отмечалось достоверной разницы по возрасту, индексу массы тела, уровню гемоглобина и основным показателям обмена железа. Исключением было повышение в группе АХЗ + ДЖ по сравнению с группой ЖДА уровней ферритина (59,0±13,7 против 15,7±9,5 мкг/л; р <0,05) и гепсидина (15,1±7,3 против 6,1±3,6 нг/мл), что является характерным признаком анемии данной этиологии.
При оценке изменений базального уровня железа у больных с ЖДА AUC колебался от 86,73 до 221,5 мкмоль×ч/л и в среднем составил 150,96±43,97 мкмоль×ч/л; CV равнялся 29,1%. У пациенток с АХЗ + ДЖ значения AUC были ниже и составили от 91,22 до 141,4 мкмоль×ч/л при среднем показателе 112,65±15,88 мкмоль×ч/л (CV 14,1%; табл. 2).
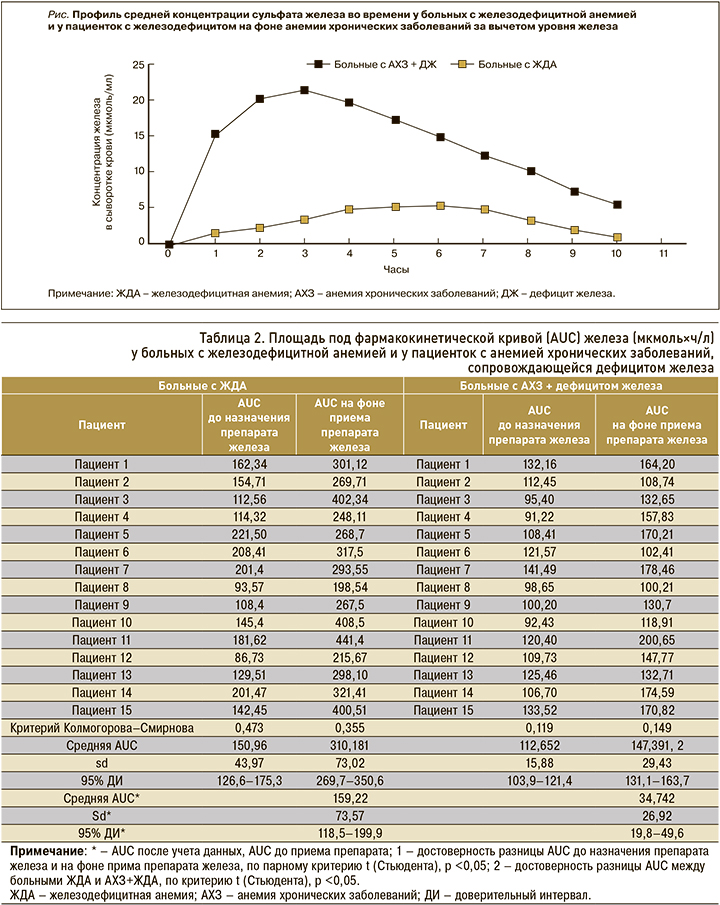
После приема 200 мг сульфата железа у больных с ЖДА AUC повысился и в среднем составил 310,18±73,02 мкмоль×ч/л (CV 23,6%). У больных с АХЗ + ЖДА AUC также повысился, его среднее значение составило 147,39±29,43 мкмоль×ч/л (CV 20%), но этот показатель было статистически значимо ниже, чем у пациенток с ЖДА (р <0,05; см. табл. 2).
Также был рассчитан средний AUC с учетом данных AUC до приема препаратов железа: он составил 159,22±73,57 мкмоль×ч/л (CV 46,2%) у больных с ЖДА против 34,72±26,92 мкмоль×ч/л (CV 77,5%) у пациенток с АХЗ + ДЖ (р <0,05; см. табл. 2).
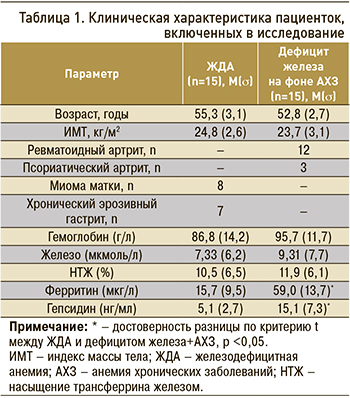
Для расчета фармакокинетических параметров препарата железа были рассчитаны AUC с учетом исходного уровня железа в крови в 08:00 до приема препарата железа. Исходные значения были вычтены из значений уровня железа во всех точках фармакокинетической кривой. Таким образом, в группе ЖДА AUC составил 156,48±51,43 мкмоль×ч/л (CV 32,86%), тогда как у больных с АХЗ + ДЖ он был статистически значимо ниже – 31,18±29,11 мкмоль×ч/л (CV 93,3%). Фармакокинетические кривые представлены на рисунке, значения Сmax, Tmax приведены в таблице 3.
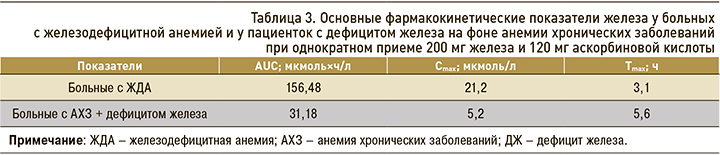
У больных с ЖДА значение максимальной концентрации (Сmax) в крови 21,2 мкмоль/л достигалось более быстро – через 3,1 ч после приема препарата; у пациенток с АХЗ + ЖДА Сmax 5,2 мкмоль/л была достигнута только через 5,6 ч.
Полученные нами результаты, как и данные других исследований, убедительно показали, что повышенная экспрессия гепсидина тесно коррелирует с биодоступностью железа как из пищи, так и из лекарственных препаратов [13, 14]. Уже при исследовании динамики базального уровня железа у больных с повышенным уровнем гепсидина отмечалось достоверное снижение AUC; эта же тенденция сохранилась и при назначении пациенткам 200 мг сульфата железа. В группе больных с АХЗ + ДЖ отмечался незначительный прирост AUC. Необходимо отметить, что в этой группе также отмечалась высокая вариабельность индивидуальных значений AUC. Итоговая фармакокинетическая кривая убедительно показала, что при повышенном содержании гепсидина трудно достигнуть необходимой компенсации дефицита железа.
ЗАКЛЮЧЕНИЕ
Повышенная экспрессия гепсидина у больных с АХЗ приводит к формированию ДЖ, в том числе в связи с уменьшением абсорбции железа из продуктов питания.
Эффективность применения препаратов железа для компенсации его дефицита у больных с повышенным уровнем гепсидина ограничена в связи со снижением биодоступности железа из пероральных препаратов.
При необходимости компенсации ДЖ у больных с АХЗ необходимо рассматривать применение парентеральных форм препаратов железа в качестве средств первой линии терапии.



