ВВЕДЕНИЕ
Кашель – наиболее частый симптом, с которым обращаются к врачу как при респираторных, так и при ряде других заболеваний. Известно, что у пациентов с этим симптомом в той или иной мере снижается качество жизни, кроме того, в силу широкой его распространенности происходит усиление экономического бремени на медицинское обслуживание со стороны здравоохранения в целом.
Подход, основанный в первую очередь на выявлении этиологии кашля, служит залогом эффективного его лечения.
Кашель – мультидисциплинарная проблема, охватывающая такие области клинической медицины, как терапия, пульмонология, кардиология, аллергология, ревматология, оториноларингология, инфекционная патология и др. При этом именно врачи общей практики и терапевты находятся на первой линии при обращении пациента в медицинское учреждение по поводу как острого, так и хронического кашля. Однако порой бывает довольно сложно определиться с четким алгоритмом действий при ведении таких пациентов.
Действительно, хронический кашель представляет собой непростую проблему в связи с большим числом потенциальных причин (и, как следствие, широким кругом дифференциально-диагностического поиска) и зачастую с несвоевременным назначением эффективной терапии [1].
Перед терапевтом и врачом общей практики стоит ряд задач:
- определить тип кашля – острый, подострый или хронический;
- установить его причину;
- выбрать диагностический объем для решения первых двух задач;
- принять решение о том, направлять ли пациента к более узким специалистам, и если да, то когда.
Международный классификатор болезней 10-го пересмотра относит кашель к рубрике R05. Различают острый (длительностью до 3 нед.), подострый (3–8 нед.) и хронический (более 8 нед.) кашель. При этом временные границы этой классификации по-прежнему вызывают споры [2].
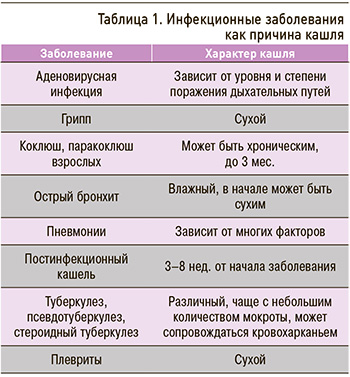
Острый кашель – наиболее распространенный симптом в первичной врачебной практике, поскольку он связан в первую очередь с острой респираторной вирусной инфекцией (ОРВИ). Однако острый кашель могут вызывать и другие причины. В частности, он может иметь бактериальную природу (табл. 1). Если вирусный кашель обычно сопутствует гриппу, парагриппу, респираторно-синтициальной вирусной, коронавирусной инфекции, кори, то кашель бактериального происхождения развивается как следствие пневмококковой, гемофильной, микоплазменной, хламидийной и других инфекций.
Острый кашель предполагает проведение дифференциальной диагностики патологий верхних и нижних дыхательных путей. Говоря об этой форме кашля, мы понимаем, что чаще всего его вызывают такие острые состояния, как ОРВИ, фарингит, тонзиллит, бронхит, пневмония. Другими возможными причинами могут служить вдыхание раздражающих веществ (пыли, дыма, газа), развитие острой аллергической реакции, бронхоспазма, коклюша, попадание инородного тела. Перед врачом амбулаторного звена всегда встает вопрос: вести ли пациента с этой патологией амбулаторно или направить в стационар?
Диагностический алгоритм при обследовании пациента, страдающего острым кашлем, в первую очередь должен быть направлен на установление его этиологии. Как уже говорилось выше, чаще всего жалобы на острый сухой кашель обусловлены ОРВИ. Врачу первичного звена следует обратить внимание на сочетание определенных клинических симптомов – ринореи, астении, гипертермии (чаще всего субфебрильной), при наличии которых выполнение какого-либо обследования чаще всего нецелесообразно.
Если же речь идет об остро возникшем кашле, которому сопутствуют фебрильная лихорадка, боль в груди, усиливающаяся на вдохе/кашле, тахипноэ, гнойная мокрота, а при проведении объективного обследования – укорочение перкуторного звука, наличие бронхиального дыхания, влажных хрипов и др., то следует включить в круг дифференциальной диагностики внебольничную пневмонию и назначить рентгенологическое исследование органов грудной клетки (ОГК). Наконец, присоединение таких жалоб, как кровохарканье, прогрессирующая одышка, потеря веса, должно насторожить врача в отношении возможного онкологического процесса и тромбоэмболии легочной артерии, что является обязательным и неотложным показанием для проведения рентгенографии ОГК [3].
Подострый кашель также называют персистирующим (поствирусным, постинфекционным). Его наличие может быть обусловлено развитием бронхиальной гиперреактивности, гиперсекрецией слизи.
Хроническим кашлем страдают до 20% взрослого населения, и эти пациенты также в первую очередь обращаются к терапевту. Распространенность этой формы кашля увеличивается с возрастом, она выше среди курильщиков и лиц, работающих в условиях загрязнения окружающей воздушной среды. При хроническом кашле спектр диагностических предположений расширяется: хронические заболевания верхних дыхательных путей, хроническая обструктивная болезнь легких (ХОБЛ), бронхиальная астма (БА), гастроэзофагеальная рефлюксная болезнь (ГЭРБ), сердечно-сосудистые, онкологические заболевания, туберкулез, интерстициальные заболевания легких, гельминтозы, реакция на прием лекарственных средств, психогенный, идиопатический кашель и др. (табл. 2).

Согласно международному документу GOLD (2023) и отечественным клиническим рекомендациям, частые ОРВИ, особенно в детстве, равно как и перенесенный туберкулез легких, относятся к факторам риска развития ХОБЛ, хотя они в этом плане и менее весомы, чем курение [4, 5]. При наличии ХОБЛ ОРВИ также оказывает влияние на течение основного заболевания. По данным исследований, не менее половины обострений ХОБЛ вызваны ОРВИ, из них около 70% – бактериальной (Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, реже другой), остальные – вирусной или смешанной микрофлорой, либо же вирусная инфекция является предшественником бактериальной [6]; например, грипп наиболее часто ассоциирован с инфекцией S. pneumoniae, H. influenzae и Staphylococcus aureus [7].
При приеме у врача-терапевта цель расспроса больного – выявление основных заболеваний как возможной причины острого и хронического кашля. Тщательно собранный анамнез помогает определить наиболее вероятную взаимосвязь ряда факторов, провоцирующих хронический кашель. Например, он чаще возникает у женщин среднего возраста. Курение выступает распространенной причиной персистирующего кашля, причем больные часто отмечают, что характер их кашля меняется после отказа от этой вредной привычки.
При ведении пациента с кашлем важно помнить следующие моменты:
- в случае острого заболевания необходимо полное излечение кашля;
- при хроническом, хорошо контролируемом заболевании, сопровождающемся небольшим легким и не обременяющем пациента кашлем, зачастую нет необходимости терапевтического вмешательства;
- при обострении хронического заболевания целесообразно лечить кашель до достижения наилучшего результата;
- в каждом случае необходимо рассматривать легочные и внелегочные причины кашля, учитывать их в ведении больного.
Симптомы, на которые следует обратить внимание при первичном обращении пациента:
- одышка (возможные причины – ХОБЛ, БА, сердечная недостаточность, туберкулез, тромбоэмболия легочной артерии и др.);
- свистящие хрипы (ХОБЛ, БА);
- снижение массы тела (рак легкого, туберкулез, ХОБЛ);
- симптомы атопии – чихание, зуд носа, глаз, диатез, атопический дерматит;
- ринорея, заложенность носа;
- изжога, горечь во рту (ГЭРБ);
- лихорадка (бактериальные инфекции, включая туберкулез, лимфома);
- ночная потливость (туберкулез, лимфома).
Полезно собрать информацию о лекарственных препаратах, принимаемых пациентом, которые потенциально могут вызвать или усилить кашель (в частности, это ингибиторы ангиотензинпревращающего фермента). Также немаловажную роль играют тщательно собранный профессиональный анамнез, информация о потенциальном воздействии пыли или химических веществ в домашних условиях.
Определенные трудности могут встречаться в дифференциальной диагностике острого бронхита от умеренного обострения интермиттирующей БА, которая часто обостряется при ОРВИ дыхательных путей. Острая БА неверно диагностируется как острый бронхит примерно у трети пациентов с острым кашлем.
При оценке характеристики кашля следует уточнить следующие аспекты.
1. Начало кашля – острое или постепенное? Внезапное начало кашля может быть связано с приступом астмы, аспирацией инородного тела.
2. Продолжительность кашля.
3. Наличие/отсутствие мокроты.
4. Внутрисуточная вариабельность кашля. Например, ночью кашель при ГЭРБ исчезает, что обусловлено закрытием нижнего эзофагеального сфинктера, и, напротив, может усиливаться в это время суток при БА и сердечной недостаточности. В случае утреннего кашля, сначала продуктивного, а затем сухого, разумно предположить назофарингит, постназальный затек, ГЭРБ.
5. Триггеры кашля: прием пищи, разговор (ГЭРБ), физическая нагрузка, контакт с аллергеном (БА).
6. «Гудящий» или «лающий» кашель, исчезающий во время сна (типичная черта психогенного кашля).
При сборе анамнеза у пациента с кашлем целесообразно уточнить следующие вопросы.
- Часто ли вы болеете гнойными синуситами и ринитами? При положительном ответе нужно исключить первичную цилиарную дискинезию.
- Часто ли вы болели пневмонией или бактериальными инфекциями? Если ответ положительный, следует исключить иммунодефицит.
- Имеется ли сезонность (кашель возникает весной/летом), наличие сопутствующих симптомов – ринита, конъюнктивита? При положительном ответе исключить поллиноз.
- Бывают ли у вас ночные приступы кашля с одышкой? Если ответ положительный, следует исключить БА, обструктивное апноэ сна.
- Были ли у вас контакты с пациентами, страдающими туберкулезом? При положительном ответе необходимо провести диаскин-тест либо квантифероновый тест.
- Курите ли вы? При положительном ответе нужно выполнить оценку функции внешнего дыхания с помощью спирометрии и пробу с бронхолитиком для исключения ХОБЛ.
- Бывает ли у вас изжога, кислый привкус во рту, тяжесть или боли в животе? При положительном ответе следует исключить рефлюкс-ассоциированные заболевания органов желудочно-кишечного тракта.
- Есть ли у вас дома пыль, плесень, домашние животные? При положительном ответе провести аллергопробы.
- Бывают ли у вас одышка / боли в груди при физической нагрузке? Если ответ положительный, необходимо исключить ишемическую болезнь сердца и астму физического усилия.
- Бывают ли у вас ощущения кома в горле, трудности при глотании, чувство давления на шею? При положительном ответе исключить заболевания щитовидной железы.
- Принимаете ли вы какие-нибудь лекарственные препараты? При положительном ответе исключить кашель, ассоциированный с приемом лекарств.
Физикальный осмотр
Результаты физикального осмотра больного с хроническим кашлем часто неспецифичны и не дают полноценной информации, например, для дифференциации обратимой бронхиальной обструкции от необратимой.
При осмотре ушей, носа, горла целесообразно обращать внимание на назальные полипы, признаки ринита, аллергического конъюнктивита, синусита, стекание назального секрета по задней стенке глотки. При осмотре шеи оценивают наличие лимфаденопатии, набухание яремной вены. В процессе осмотра, пальпации, перкуссии, аускультации грудной клетки выявляют признаки, свидетельствующие в пользу ХОБЛ, БА, пневмонии, интерстициального фиброза, бронхоэктазов. Например, грубые крепитирующие хрипы могут быть важным признаком бронхоэктазов, тогда как распространенная крепитация по типу хруста снега или целлофанового звука на высоте вдоха типична для интерстициальных заболеваний легких.
Какие исследования следует назначить при кашле?
В случае острого кашля всем пациентам выполняют общий анализ крови и рентгенографию ОГК в двух проекциях. При хроническом кашле к этим исследованиям добавляется спирометрия с бронходилатационным тестом. Компьютерную томографию (КТ) ОГК в острых ситуациях назначают лишь при подозрении на вирусную пневмонию и ТЭЛА. При хроническом кашле КТ рекомендуют в том случае, когда выявляются отклонения в ходе аускультации и перкуссии легких, а при проведении рентгенографии ОГК нет изменений или они есть, но требуют уточнения.
Другие исследования назначают в зависимости от конкретной клинической ситуации. В частности, при наличии диспепсических жалоб пациенту потребуется эзофагогастродуоденоскопия, при подозрениях на БА и ХОБЛ – спирометрия (проба с бронхолитиком), другие методы обследования. Если по результатам осмотра и многочисленных тестов причина кашля не установлена, следует направить пациента к неврологу для исключения нейрогенного кашля.
Причины, по которым терапевт направляет пациентов с кашлем к пульмонологу
1. Диагноз не ясен.
2. Диагноз известен, но есть трудности в лечении (пневмония тяжелого течения, например деструктивная или с выраженным распространенным фиброзом, после стационарного этапа лечения и т. д.).
3. Заболевание имеет тяжелое течение, сопровождается развитием осложнений (например, тяжелая неконтролируемая БА, ХОБЛ с бронхоэктазами, с дыхательной недостаточностью).
4. Заболевание находится вне компетенции терапевта (например, идиопатический легочный фиброз, лимфангиолейомиоматоз).
5. Есть трудности с ведением мультиморбидного пациента (например, сочетание кашля с сердечно-сосудистыми заболеваниями, сахарным диабетом, ожирением и синдромом обструктивного апноэ сна).
При всей важности верного диагноза его установление лишь половина пути. Принимая во внимание защитный физиологический механизм кашля, обеспечивающий удаление избыточного бронхиального секрета, правильнее говорить о стратегии управления кашлем, а именно об устранении кашля как патологического симптома.
МЕТОДЫ УСТРАНЕНИЯ И УПРАВЛЕНИЯ КАШЛЕМ
Для устранения и управления кашлем используются немедикаментозные и фармакологические методы. К первым относятся отказ от курения (достоверно снижающий частоту обострений и прогрессирования ХОБЛ и хронического бронхита), обеспечение достаточной гидратации, увлажнение воздуха в помещении, ограничение контакта с аллергенами и отмена препаратов, потенциально провоцирующих кашель.
Фармакологические методы применяют дифференцированно, в зависимости от причин, вызывающих кашель. При этом они подразделяются на две группы.
1. Терапия, повышающая эффективность кашля, – протуссивная терапия.
Такая терапия, в свою очередь, подразделяется на медикаментозную (мукоактивные препараты, изотонические и гипертонические растворы натрия хлорида, бронхолитики) и немедикаментозную (физиотерапевтические методы: контролируемый кашель, хаффинг, постуральный дренаж, дыхательные техники, мануальная перкуссия и вибрация, аппаратная поддержка бронхиального клиренса; природные факторы – спелео-, галотерапия).
2. Терапия контролирующая (предупреждающая или устраняющая кашель) – антитуссивная терапия:
- лекарственные средства центрального действия, вызывающие торможение кашлевого центра (ненаркотические и наркотические противокашлевые препараты – кодеин, бутамирата цитрат);
- препараты периферического действия, снижающие чувствительность афферентных рецепторов за счет действия на слизистую оболочку дыхательных путей (преноксидиазин, леводропропизин);
- препараты комбинированного действия (комбинация антител к брадикинину, гистамину, морфину, другие комбинации);
- лекарственные средства, влияющие на причины и механизмы воспаления (антибиотики, глюкокортикостероиды, Н1-блокаторы).
Антигистаминные препараты не следует применять при инфекционных заболеваниях. В рандомизированных клинических исследованиях они не облегчали симптомы кашля, не влияли на продолжительность и частоту его приступов. Кроме того, они могут вызывать побочные эффекты: седацию или, наоборот, повышенную возбудимость, угнетение дыхания и галлюцинации.
Противокашлевые средства. В настоящее время не рекомендуют использовать в рутинной клинической практике кодеин и декстрометорфан для лечения кашля, обусловленного ОРВИ. Причина – отсутствие рандомизированных клинических исследований, демонстрирующих эффективность и безопасность этих препаратов. При этом существуют доказательства, что они могут стать причиной опасных побочных реакций, например респираторного дистресс-синдрома.
Пациентам можно назначать противокашлевые препараты центрального действия, не содержащие кодеин, с действующим веществом бутамират. Они не вызывают наркотическую зависимость, не угнетают дыхательный центр и моторику желудочно-кишечного тракта. Но их прием предусмотрен при наличии строгих показаний – подавление сухого мучительного кашля при коклюше, гриппе и т. д.
Отхаркивающие и муколитики очень широко используются в России по сравнению с другими странами (рис. 1, табл. 3). Специалисты Клиники Мейо (Mayo Clinic) не рекомендуют назначать безрецептурные отхаркивающие средства (гвайфенезин) или муколитики (ацетилцистеин, бромгексин, летостеин) без соответствующих показаний. Кроме того, следует помнить об их возможных побочных эффектах, включающих бронхоспазм, желудочно-кишечные расстройства и лихорадку.

Бронходилататоры не рекомендуются для лечения кашля у пациентов, не страдающих БА. При этом заболевании, согласно последним рекомендациям GINA, необходимо назначать бронходилататоры, которые купируют острый приступ обструкции; при этом базисную терапию не отменяют.
Ингаляционные бронходилататоры у пациентов без БА можно использовать для купирования бронхиальной обструкции. Чаще всего применяют селективные β2-адреномиметики короткого/длинного действия или их комбинации с антихолинергическими препаратами, например сальбутамолом, фенотеролом.
Муколитики уменьшают вязкость мокроты за счет разрушения третичной структуры бронхиального секрета, преимущественно влияют на гелевый слой бронхиального секрета, оказывая мукоактивное действие в просвете бронхов. Количество бронхиального секрета при этом существенно не изменяется [8, 9].
Первым производным цистеина со свободной тиоловой группой, разрывающей дисульфидные связи, стал в свое время N-ацетилцистеин. Флуимуцил (оригинальный N-ацетилцистеин) – плейотропный препарат с гетерогенными фармакологическими характеристиками. Он препятствует экспрессии цитокинов, обладающих провоспалительным действием (интерлейкинов 1β, 6 и 8, фактора некроза опухоли-альфа и др.), подавляет активность транскрипционного фактора NF-kB, играющего важную роль в развитии воспаления [8, 10, 11]. Фармакодинамический спектр N-ацетилцистеина включает муколитическое, противовоспалительное, антиадгезивное, детоксикационное, антиоксидантное действие [11–15]. Флуимуцил активен в отношении всех типов бронхиального секрета и может применяться при кашле, вызванном как вирусными, так и бактериальными инфекциями.
Муколитический эффект N-ацетилцистеина реализуется за счет расщепления дисульфидных связей мукополисахаридов мокроты, увеличения секреция менее вязких сиаломуцинов (гель-слой), что приводит к снижению вязкости мокроты, улучшению мукоцилиарного клиренса и облегчает откашливание без увеличения объема мокроты [11, 16].
Антиадгезивное действие N-ацетилцистеина заключается в том, что он препятствует адгезии бактерий к эпителию слизистой дыхательных путей, расщепляет биопленки и препятствует их формированию (рис. 2) [12, 17].

В таблице 4 приведена сравнительная активность некоторых муколитиков и мукокинетиков при появлении различных видов мокроты. Ацетилцистеин (Флуимуцил®) продемонстрировал активность в отношении как слизистой, слизисто-гнойной, так и гнойной мокроты [18].
На рисунке 3 схематично представлены плейотропные эффекты N-ацетилцистеина, которые также могут оказаться полезными при лечении пациента с кашлем [19, 20].
Необходимость антибактериальной терапии – ключевой вопрос в лечении острых инфекционных заболеваний верхнего отдела дыхательных путей. Считается, что только 0,2–2% случаев ОРВИ у взрослых осложняются вторичной бактериальной инфекцией [21, 22].
Даже в случае такого относительно небольшого количества пациентов с развитием бактериальных осложнений в повседневной практической деятельности врача первичного звена и более узких специалистов (пульмонолога, оториноларинголога) нередко встречаются ситуации, когда сложно однозначно констатировать наличие показаний к назначению системной антибактериальной терапии у конкретного пациента, указанные в национальных клинических руководствах. Важно помнить, что среди критериев качества медицинской помощи пациентам с инфекционно-воспалительными заболеваниями верхних дыхательных путей, обозначенных в актуальных российских клинических рекомендациях, назначение системных антибактериальных препаратов при наличии соответствующих показаний присутствует в обязательном порядке [23, 24].
К основным клиническим ситуациям, наличие которых дает основания врачу назначить антибактериальные препараты, относятся:
- «красные флаги» при кашле (цианоз, снижение сатурации при пульсоксиметрии, кашель с кровянистой или розовой мокротой, боль в груди, дыхательная недостаточность, выраженная интоксикация);
- установленный диагноз пневмонии (в первые 8 ч от момента диагностики пневмонии следует дать первую дозу антибактериального препарата);
- сопутствующие хронические заболевания;
- иммунодефицит или иммуносупрессия;
- муковисцидоз;
- госпитализации в стационар в течение года;
- сахарный диабет 1-го или 2-го типа;
- застойная сердечная недостаточность в анамнезе;
- прием пероральных форм кортикостероидов на момент заболевания (> 10 мг/сут. по преднизолону в ближайшие 2 нед.).
В последние годы выбор подходящих противомикробных средств стал более сложным, так как у многих из преобладающих бактериальных патогенов развилась устойчивость к широко используемым антибиотикам, на фоне которой врачи назначают повторные курсы антибиотиков широкого спектра действия, тем самым создавая селективное давление, способствующее возникновению еще большей резистентности к противомикробным препаратам [25].
Считается, что практически при любой ОРВИ в патологический процесс в той или иной степени вовлекается слизистая оболочка околоносовых пазух, поэтому правомочно говорить о развитии острого риносинусита (ОРС), который зачастую также сопровождается таким симптомом, как кашель. Наиболее частыми вирусами, вызывающими ОРС, являются риновирусы, вирусы гриппа и парагриппа, респираторно-синцитиальные, аденовирусы, коронавирусы, бокавирусы, метапневмовирусы и др. Спектр возбудителей бактериального ОРС остается относительно постоянным, но чаще всего у пациентов идентифицируют S. pneumoniae и H. influenzae (суммарно 70–75%) [23, 26].
Стратегия традиционного лечения ОРС в США делает упор на использование антибиотиков сразу у 85–98% пациентов, несмотря на то что в большинстве случаев заболевание имеет вирусное происхождение, и лишь в незначительном числе случаев развивается вторичная бактериальная инфекция [27].
Принципиальный вопрос – переход вирусного ОРС в бактериальный. Не существует абсолютных критериев, позволяющих в рутинной клинической практике со 100% точностью поставить диагноз бактериального ОРС. Определенную сложность в дифференциальную диагностику привносит и тот факт, что вирусный и бактериальный риносинуситы зачастую «накладываются» друг на друга. Суммируя данные последних международных согласительных документов по проблеме ОРС, в качестве основных критериев постановки диагноза «острый бактериальный синусит» можно выделить длительность заболевания более 10 дней или ухудшение состояния после 5-го дня от начала ОРВИ. Клиническими признаками ОРС бактериальной этиологии могут быть гнойное отделяемое, односторонний болевой синдром в проекции придаточных пазух и ухудшение общего самочувствия. Лабораторные маркеры бактериальной инфекции включают повышения уровня С-реактивного белка и прокальцитонина. Использование указанных биомаркеров позволяет избежать необоснованного назначения антибактериальной терапии [21, 28].
В российских клинических рекомендациях, представленных экспертами Национальной медицинской ассоциации оториноларингологов России, рассматривается возможность применения антибактериальных препаратов местного действия в качестве монотерапии или в комбинации с противовоспалительными препаратами и отхаркивающими муколитическими препаратами в лечении пациентов с легким течением ОРС, имеющих отдельные косвенные признаки бактериального воспаления, в частности ринорею гнойного характера. Врач должен оценивать клинический эффект применения топических антибактериальных препаратов через 3–4 дня от начала терапии, при отсутствии положительного эффекта рассматривается вопрос о необходимости назначения антибактериальных препаратов системного действия [23]. Определенный интерес вызывает комбинированный препарат тиамфеникола и N-ацетилцистеина. Как и остальные лекарственные средства для местного применения в полости носа, он имеет низкий риск развития нежелательных явлений. При этом пневмококк, выступающий одним из основных возбудителей бактериального риносинусита, в России сохраняет хорошую чувствительность к амфениколам, к которым, в частности, относится тиамфеникол. Также амфениколы имеют высокую активность в отношении гемофильной палочки [29, 30]. Есть данные и о прямом антибактериальном действии N-ацетилцистеина, что допускает вероятность его синергического действия с тиамфениколом [31–33].
В качестве демонстрации модели пациента, которому с целью терапии ОРС в амбулаторной практике целесообразно назначить комбинированный препарат тиамфеникола и N-ацетилцистеина, рассмотрим клинический пример.
ОПИСАНИЕ КЛИНИЧЕСКОГО СЛУЧАЯ
Пациент Д., 57 лет, обратился в поликлинику к терапевту. При первичном осмотре предъявлял жалобы на повышение температуры тела до 37,6 °С, недомогание, заложенность носа, ночной кашель, слизисто-гнойное отделяемое из носа, боль в проекции гайморовых пазух.
Данные анамнеза заболевания: за 7 дней до обращения к врачу появились насморк, слизистые выделения из носа, через 4 дня к ним присоединились повышение температуры тела до 37,6 °С, недомогание, ночной кашель, слизисто-гнойное отделяемое из носа, боль в проекции гайморовых пазух.
Данные анамнеза жизни: из перенесенных респираторных заболеваний за последний год – два эпизода ОРВИ с клинической картиной острого назофарингита. Системные и топические антибактериальные препараты пациент за последний год не принимал. По официальным данным, около года назад перенес COVID-19 в легкой форме. Страдает артериальной гипертензией II стадии, 1-й степени в течение 10 последних лет, принимает периндоприл 2 мг + индапамид 0,625 мг в дозе 10 мг/сут., на фоне антигипертензивной терапии достигнут устойчивый контроль уровня артериального давления (АД). Курит в течение 30 лет, по 1 пачке сигарет в сутки, индекс курильщика – 30 пачка/лет. Около 10 лет отмечает привычное отделение небольшого количества слизистой мокроты по утрам. Аллергологический анамнез: без особенностей.
Объективные данные: общее состояние удовлетворительное, кожные покровы телесного цвета, чистые, повышенной влажности. При аускультации легких – дыхание с жестким оттенком, хрипов нет. Частота дыхательных движений (ЧДД) 16 в минуту, SatO2 96%. Частота сердечных сокращений 89 в минуту, ритм правильный, АД 138/88 мм рт. ст. Язык обложен беловатым налетом, влажный. Со стороны других органов и систем без особенностей. Индекс массы тела 32 кг/м2.
Риноскопия (рис. 4): визуализируются умеренный отек слизистой оболочки полости носа, скопление вязкого слизисто-гнойного секрета в просвете общего среднего и нижнего носового ходов, больше в задних отделах полости носа. Перегородка носа – по средней линии, носовые раковины – без особенностей.

Пальпация и перкуссия проекции околоносовых пазух умеренно болезненны.
Фарингоскопия: незначительное стекание секрета по задней стенке глотки слизисто-гнойного характера, в остальном – без особенностей.
Отоскопия: патологических изменений не выявлено.
Назначены следующие диагностические мероприятия:
- рентгенография околоносовых пазух;
- рентгенография ОГК;
- клинический анализ крови;
- биохимический анализ крови;
- оценка тяжести симптомов по визуально-аналоговой шкале (ВАШ).
Результаты исследований
Клинический анализ крови: эритроциты – 4,3 × 1012/л; гемоглобин – 138 г/л; лейкоциты – 9 × 109/л; эозинофилы – 3%; нейтрофилы (палочкоядерные) – 5%; нейтрофилы (сегментоядерные) – 62%; лимфоциты – 24%; моноциты – 6%, скорость оседания эритроцитов – 11 мм/ч. Заключение: показатели в пределах нормы.
Биохимический анализ крови: глюкоза – 4,7 ммоль/л; общий белок – 75 г/л; С-реактивный белок – 5 мг/л; мочевина – 4,3 ммоль/л; креатинин – 97 мкмоль/л; билирубин общий – 6,3 мкмоль/л; аланинаминотрансфераза – 20 МЕ/л; аспартатаминотрасфераза – 18 МЕ/л; прокальцитонин – 0,02 нг/мл. Заключение: показатели в пределах нормы.
Рентгенография околоносовых пазух: незначительные пристеночные утолщения в верхнечелюстных пазухах (рис. 5).

Рентгенография органов грудной клетки: усиление легочного рисунка, уплотнение аорты, небольшое увеличение тени левого желудочка, очаговых и инфильтративных изменений нет.
Оценка тяжести симптомов по ВАШ: 7 баллов – средняя тяжесть заболевания. Значение по ВАШ более 5 баллов свидетельствует о неблагоприятном влиянии заболевания на качество жизни пациента.
По результатам оценки жалоб, анамнеза, результатов лабораторных и инструментальных исследований пациенту был поставлен диагноз ОРС.
Было рекомендовано:
- орошение полости носа изотоническим раствором стерильной морской воды;
- назальный спрей с глюкокортикостероидом;
- назальный спрей с ацетилцистеином;
- назальный деконгестант (при необходимости);
- отказ от курения;
- контрольный осмотр через 3–4 дня.
Контрольный осмотр у терапевта на 4-й день лечения: отмечалось улучшение носового дыхания, но при этом изменился характер отделяемого из полости носа (гнойный), появился кашель с мокротой зеленого цвета, увеличилось количество мокроты. Аускультативно – рассеянные сухие хрипы в легких. Терапия прежняя. Пациент был направлен на консультацию пульмонолога.
Консультация пульмонолога через 14 дней от начала заболевания и через 7 дней после первой консультации терапевта: жалобы на кашель со слизисто-гнойной мокротой в течение дня, уменьшение заложенности носа, облегчение очищения полости носа, но сохранение отделяемого гнойного характера, слабость, потливость, вечерний субфебрилитет до 37,1–37,2 °С.
При аускультации легких – бронхиальное дыхание, рассеянные сухие хрипы средней тональности, на форсированном выдохе со свистящим оттенком, форсированный выдох несколько удлинен. ЧДД 18 в минуту, SatO2 96%. Данные оценки портативным спирометром на приеме: объем форсированного выдоха за 1-ю секунду (ОФВ1) 68%, форсированная жизненная емкость легких (ФЖЕЛ) 94% от должного, ОФВ1/ФЖЕЛ 67%.
Диагноз: хронический бронхит, обострение средней степени тяжести с обструктивным синдромом. ХОБЛ? Острый риносинусит средней степени тяжести.
Лечебные мероприятия:
- соблюдение домашнего режима (минимизация рисков очередного эпизода вирусной суперинфекции);
- орошение полости носа изотоническим раствором стерильной морской воды (оптимизация туалета полости носа и улучшение эффекта от последующего использования местных препаратов);
- ингаляции через небулайзер раствора ипратропия бромида 0,25 мг/мл с фенотеролом 0,5 мг/мл по 2 мл 2 р./сут. (бронхолитическое действие, улучшение мукоцилиарного клиренса);
- ингаляции препарата Флуимуцил®-антибиотик ИТ (тиамфеникола глицинат ацетилцистеинат) по 250 мг утром и днем в течение 6 дней (антибактериальное действие);
- мометазона фуроат по 50 мкг 2 р./сут. интраназально (суточная доза 200 мкг) курсом на 14 дней (противовоспалительный эффект);
- отказ от курения;
- контрольный осмотр через 6 дней;
- дообследование – спирометрия с бронходилатационным тестом (сальбутамол 400 мкг) после выздоровления;
- отказ от курения;
- контрольный осмотр для оценки эффективности проводимого лечения.
Результаты
Через 6 дней лечения на 3-м контрольном осмотре у терапевта пациент наблюдалось улучшение общего самочувствия, улучшение носового дыхания, изменение характера и количества отделяемого (стало прозрачным и водянистым, объем его небольшой), уменьшение кашля, мокрота носит слизистый характер.
Таким образом, при контрольном осмотре пациент жалоб не предъявлял, отмечал существенное уменьшение респираторных симптомов, нормализацию общего самочувствия. По завершении ингаляционной терапии комплексным препаратом, содержащим тиамфеникола глицинат ацетилцистеинат (Флуимуцил®-антибиотик ИТ), гнойные выделения из носа прекратились в течение 2 дней, носовое дыхание полностью восстановилось, кашель носит незначительный характер, мокрота (слизистая) появляется редко в небольшом количестве. При оториноларингологическом осмотре не было выявлено каких-либо патологических признаков. Было констатировано выздоровление от острого риносинусита, обострения хронического бронхита. Пациент был направлен на спирометрию с бронходилатационным тестом и последующей повторной консультацией пульмонолога.
Установлено диспансерное наблюдение с диагнозом «хронический бронхит». Проведена беседа о необходимости полного отказа от курения, пациент направлен в кабинет медицинской профилактики.
Информированное согласие
От пациента было получено письменное добровольное информированное согласие на публикацию фотографий, результатов обследования и лечения.



