ВВЕДЕНИЕ
Сахарный диабет (СД) – одно из самых распространенных и сложных метаболических заболеваний, представляющее собой серьезную медико-социально-экономическую проблему во всем мире [1]. Основной причиной заболеваемости и смертности у соответствующих больных остаются диабетические осложнения. К числу наиболее частых и потенциально опасных состояний при СД относится нарушение функции почек.
Диабетическая нефропатия (ДН) – специфическое поражение почек при СД, неизбежно ведущее к терминальной стадии почечной недостаточности (ТСПН) и существенно повышающее риск смерти как от всех причин, так и от сердечно-сосудистых заболеваний (ССЗ) [2]. По данным эпидемиологических исследований, число пациентов с СД и хронической болезни почек (ХБП) увеличивается пропорционально повышению распространенности самого диабета, что в значительной степени обусловлено ожирением, гиподинамией, эпидемией СД 2-го типа (СД 2) и ростом заболеваемости СД 1-го типа (СД 1) [3].
ХБП – это надназологическое понятие, обозначающее прогрессирующее ухудшение почечной функции. В соответствии с действующими рекомендациями KDIGO (Kidney Disease Improving Global Outcomes) ХБП определяется при наличии любых клинических и морфологических признаков, указывающих на повреждение почек и/или при снижении скорости клубочковой фильтрации (СКФ) менее 60 мл/мин/м2 вне зависимости от причин в течение 3 и более месяцев [4]. Несмотря на широкое применение стандартных методов лечения в течение последних нескольких десятилетий, темпы распространенности и прогрессирования ДН до терминальной стадии заболевания остаются высокими. Так, в исследовании Sui Z. et al. ДН занимала 3-е место (27,1%) среди причин ХБП после хронического гломерулонефрита (36,8%) и гипертонического гломерулосклероза (28,5%) [5].
Согласно данным Федерального регистра СД за 2013–2016 гг., частота регистрации ХБП у пациентов с СД 1 и СД 2 составила 23 и 6,9% соответственно, а доля больных с очень высоким риском (стадии ХБП С3а–5, А2–3) равнялась 6,7% при СД 1 и 4,4% при СД 2 [6]. Аналогичным образом в другом крупном исследовании, которое включало более 1 млн пациентов с СД 2, наличие ДН было связано с повышенным риском сердечно-сосудистых катастроф и общей смертности. Также в работе Afkarian M. et al. было показано, что общий риск смертности у таких больных на 23% выше, чем у пациентов без диабета и ХБП [7]. Таким образом, наличие ХБП у пациентов с СД имеет особое клиническое значение, поскольку ассоциировано с повышенным риском смертности как от всех причин, так и от ССЗ независимо от других факторов риска.
В данной работе основное внимание уделено патофизиологии ХБП при СД 2, подходам к диагностике, а также современным и новым терапевтическим вмешательствам.
ПАТОФИЗИОЛОГИЯ ХРОНИЧЕСКОЙ БОЛЕЗНИ ПОЧЕК ПРИ САХАРНОМ ДИАБЕТЕ
Патогенез развития и прогрессирования ХБП сложный и многофакторный [8, 9]. На сегодняшний день предложено несколько теорий развития ДН, из которых наиболее изучены гемодинамическая и метаболическая.
Открытие доктором В.М. Бреннером феномена гиперфильтрации и внутриклубочковой гипертензии стало отправной точкой в понимании патофизиологии ДН [10]. Ведущую роль в развитии этого механизма отводят сверхвысокой активности ренин-ангиотензин-альдостероновой системы (РААС) и ее главному эффекторному пептиду – ангиотензиогену II (АТ II), почечная концентрация которого в 1000 раз превышает его содержание в плазме. АТ II оказывает провоспалительное и профибротическое действие, повышает активность симпатической нервной системы, увеличивает канальцевую реабсорбцию натрия и хлоридов, секрецию альдостерона и антидиуретического гормона [11, 12]. Активация АТ II способствует повышению кровяного давления внутри клубочков в результате дисбаланса тонуса приносящей и выносящей артериол, что, в свою очередь, приводит к развитию «гидравлического удара», запускающего процессы склероза. Данный феномен способствует нарушению архитектоники и проницаемости базальной мембраны клубочка, что в последующем влечет за собой структурные и функциональные изменения в почках [13]. Кроме того, АТ II запускает ряд клеточных сигнальных каскадов, включая МАРК (p38 и JNK) и PKC-β (рrotein kinase C beta-type), которые опосредуют клеточный ответ путем активации факторов транскрипции, таких как NF-κB (nuclear factor-κB). Активация этих путей вызывает гломерулярную эндотелиальную дисфункцию и характеризуется снижением внутрипочечной продукции оксида азота (NO), увеличением образования активных форм кислорода (АФК) и ингибитора активатора плазминогена-1 (PAI-1), провоспалительных цитокинов, в том числе фактора некроза опухоли альфа и молекул адгезии, таких как межклеточные (ICAM) и сосудистые молекулы клеточной адгезии (VCAM), которые, в свою очередь, поддерживают хроническое воспаление низкой интенсивности. Активация ICAM-1 на эндотелиальных клетках способствует миграции циркулирующих мононуклеарных клеток в почки с последующим развитием местного воспаления и повышением продукции провоспалительных и профибротических цитокинов, АФК и антиангиогенных факторов, приводя к окислительному стрессу, клеточному повреждению, прогрессирующему фиброзу и снижению СКФ (рис. 1) [14–16].
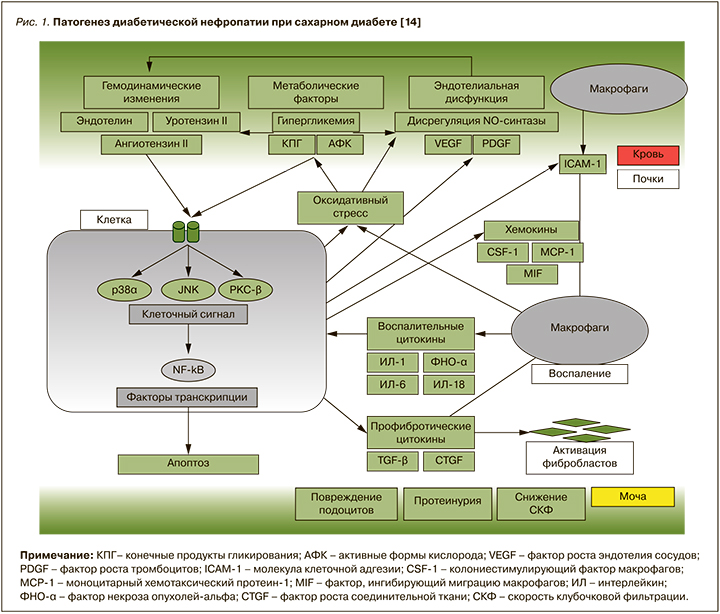
Таким образом, патогенное действие АТ II при СД обусловлено не только его мощным вазоконстрикторным действием, но и стимуляцией синтеза провоспалительных медиаторов, цитокинов, хемокинов, факторов роста, которые в совокупности провоцируют развитие гломерулосклероза, тубулоинтерстициального фиброза, что в итоге вызывает почечную недостаточность.
В метаболической теории ДН ведущая роль отводится хронической гипергликемии, в условиях которой увеличивается поступление глюкозы в проксимальные отделы канальцев почек. Следствием этого становится неадекватное возрастание реабсорбции глюкозы и натрия с участием натрий-глюкозных котранспортеров-2 (SGLT2, НГЛТ-2) в проксимальных канальцах. В ответ на снижение поступления натрия в дистальные отделы нефрона и стимуляцию юкстагломерулярного аппарата происходит расширение приносящей и сужение выносящей артериолы с развитием внутриклубочковой гиперфильтрации [17, 18].
Следующей стадией поражения почек при СД является развитие микроальбуминурии, обусловленное подоцитарной дисфункцией. Гипергликемия способствует повреждению подоцитов через ряд биохимических нарушений, таких как:
- неферментативное связывание глюкозы с белками, липидами и нуклеиновыми кислотами с последующим образованием обратимых (шиффовы основания), частично обратимых (продукты Амадори) и, наконец, необратимых конечных продуктов гликирования (КПГ), способствующих окислительному стрессу и экспрессии провоспалительных цитокинов и факторов роста;
- активация диацилглицерол-протеинкиназы C многофункционального семейства энзимов, ответственных за целый ряд патофизиологических изменений в сосудистой стенке;
- активация полиолового пути, при котором альдозоредуктаза превращает избыток глюкозы в сорбитол с последующим образованием фруктозы под действием сорбитолдегидрогеназы.
Под действием этих факторов клетки клубочков (подоциты) подвергаются структурно-функциональным изменениям с деструкцией межподоцитарной щелевой диафрагмы. Подоцитарное повреждение сопровождается нарушением селективной проницаемости клубочкового фильтра, что обусловливает потерю белка с мочой и в дальнейшем снижение функции почек [19]. Таким образом, развитие и прогрессирование ХБП обусловлено взаимосвязанными патофизиологическими процессами: метаболическими, гемодинамическими нарушениями, воспалением и фиброзом. Эти механизмы приводят к структурным изменениям в нефроне и связаны с альбуминурией, артериальной гипертензией (АГ), снижением СКФ и увеличением сердечно-сосудистых событий и смертности.
КЛИНИЧЕСКАЯ ФЕНОМЕНОЛОГИЯ ХРОНИЧЕСКОЙ БОЛЕЗНИ ПОЧЕК У ПАЦИЕНТОВ С САХАРНЫМ ДИАБЕТОМ
Согласно классической концепции течения ДН, увеличение экскреции альбумина с мочой предшествует снижению СКФ, и в связи с этим альбуминурия считается ранним маркером ХБП. Однако в 1992 г. эта клиническая парадигма впервые подверглась сомнению после исследования Lane P.H. et al., которые в своей работе описали пациентов с ХБП и нормоальбуминурией [20]. Также по данным исследования UKPDS (n=5102) было продемонстрировано, что примерно у 50% пациентов с СД 2 при расчетной СКФ (рСКФ) ≤60 мл/мин/1,73 м2 не наблюдалась повышенная экскреция альбумина с мочой (ЭАМ) [20]. В работе DEMAND (Developing Education on Microalbuminuria for Awareness of Renal and Cardiovascular Risk in Diabetes) 17% больных имели нормоальбуминурический вариант течения ХБП (НА-ХБП) [21]. В другом исследовании, выполненном в США, доля таких пациентов составила 33% [22]. Эти результаты согласуются с данными Afkarian M. et al., которые показали, что, несмотря на увеличение числа больных ХБП со сниженной СКФ с 1994 по 2014 г., за тот же период наблюдалось снижение роста пациентов с повышенной ЭАМ [23, 24]. Таким образом, развитие и прогрессирование снижения почечной функции может происходить независимо от альбуминурии.
Результаты приведенных исследований привлекли пристальное внимание ученых, и за последние десятилетия был проведен ряд новых клинических исследований, которые позволили описать клинический «портрет» ХБП у пациентов с СД в зависимости от формы его течения. Так, в работе Penno G. et al. было показано, что нормоальбуминурический вариант ДН чаще встречался у лиц женского пола, причем такие пациенты имели более низкие значения уровня гликированного гемоглобина (HbA1c) и систолического артериального давления (САД) по сравнению с больными, имеющими альбуминурическую форму ДН. Также среди больных с СКФ менее 30 мл/мин/1,73 м2 нормоальбуминурию чаще сохраняли женщины и некурящие лица [25, 26].
В другом исследовании мужской пол, плохой контроль гликемии, низкий уровень липопротеидов высокой плотности (ЛПВП), ожирение и курение были независимо ассоциированы с альбуминурией и микрососудистыми осложнениями, тогда как женский пол и высокий уровень креатинина были связаны с НА-ХБП и макрососудистыми осложнениями [27].
Данные зарубежных исследований созвучны с отечественными. К примеру, в работе В.В. Климонтова с соавт. развитие альбуминурии без снижения СКФ было ассоциировано с мужским полом, курением, недостаточным контролем гликемии и ожирением абдоминального типа, НА-ХБП – с женским полом, возрастом старше 65 лет, большим стажем СД и приемом диуретика, а классическая модель ХБП – с длительным анамнезом СД 2 и применением блокаторов медленных кальциевых каналов [28].
Учитывая тот факт, что ССЗ служат основной причиной смертности у пациентов с СД, отдельного внимания заслуживают исследования, направленные на оценку взаимосвязи альбуминурии и рСКФ (нарушенной функции почек) с риском развития кардиоваскулярных событий. Результаты популяционной программы KEEP (Kidney Early Evaluation Program) продемонстрировали, что ХБП выступает значимым предиктором преждевременной смерти от болезней кровообращения (инфаркта миокарда или инсульта) у мужчин до 55 лет и женщин до 65 лет (отношение шансов (ОШ) 1,44; 95% доверительный интервал (ДИ): 1,27–1,63) [29]. Аналогичным образом данные шведского регистра за 2003–2006 гг. с последующим наблюдением в течение 5,7 лет (n=66 065) подтвердили роль альбуминурии и СКФ как независимых факторов риска ССЗ. Стоит упомянуть, что в этой работе также были выявлены различия в структуре факторов риска ССЗ у больных c СД 2 в зависимости от варианта течения ДН. Дислипидемия, курение и контроль гликемии (уровень HbA1c) были ассоциированы с риском развития сердечно-сосудистых событий у пациентов с ХБП и альбуминурией, в то время как у больных с НА-ХБП риск осложнений зависел от АД и не был связан с контролем глюкозы. Наиболее неблагоприятным прогностическим признаком оказалось сочетание сниженной СКФ и наличие макроальбуминурии [30]. На основании результатов нескольких исследований было сделано предположение, что классическая альбуминцентрическая модель ХБП – это проявление микроангиопатии, а нормоальбуминурический вариант – результат поражения крупных сосудов или перенесенных эпизодов острого почечного повреждения.
Таким образом, у больных СД с повышенной альбуминурией наиболее значимыми факторами сердечно-сосудистых осложнений являются уровень HbA1c, курение и выраженность гиперлипидемии, тогда как у больных с НА-ХБП риск таких осложнений зависел главным образом от уровня АД и не был связан с качеством контроля гликемии.
КЛАССИФИКАЦИЯ И ДИАГНОСТИКА ХРОНИЧЕСКОЙ БОЛЕЗНИ ПОЧЕК
ХБП определяется как нарушение структуры или функции почек, сохраняющееся более 3 мес. Маркерами почечного повреждения служат СКФ менее 60 мл/мин/1,73 м2; альбуминурия (скорость экскреции альбумина ≥30 мг за 24 ч или отношение альбумина к креатинину (А/кр) мочи ≥30 мг/г); изменения мочевого осадка при визуализирующих методах исследования, гистологические изменения; трансплантация почки в анамнезе [31, 32].
Современная классификация ХБП, независимо от этиологии, основана на рСКФ и скорости экскреции альбумина. СКФ отражает количество функционирующих нефронов, в то время как альбуминурия является маркером структурного повреждения почек [33]. Итак, ХБП делится на 5 стадий в соответствии с СКФ и на 3 стадии в зависимости от альбуминурии (табл.). При этом 3-я стадия по рСКФ подразделяется на подгруппы 3а и 3б; это связано с тем, что при стадиях 1–3а сердечно-сосудистые факторы риска доминируют над почечными, а при ХБП стадий 3б–5-й вероятность развития ТСПН и почечной смерти выше [31].
Следует помнить, что стратификация риска сердечно-сосудистых осложнений при ХБП имеет свои особенности. У больных с ХБП рекомендуется применение системы оценки риска терминальной почечной недостаточности и кардиоваскулярных осложнений, основанной на двух показателях – уровне СКФ и категории альбуминурии (см. табл.).
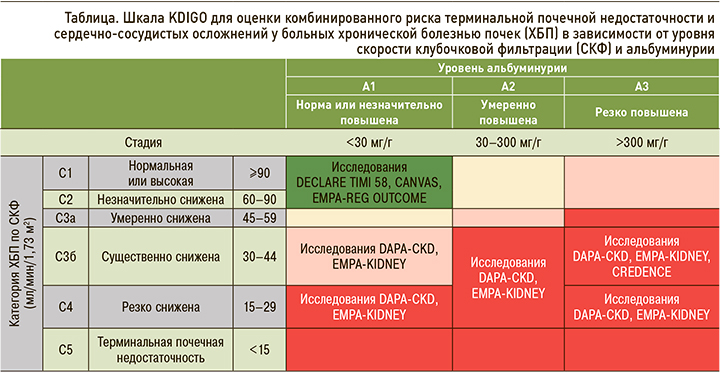
Согласно российским алгоритмам специализированной медицинской помощи больным СД и гайдлайнам KDIGO, рСКФ рекомендуется оценивать методом CKD-EPI на основании возраста, пола и уровня креатинина в крови. Однако, учитывая, что креатинин является продуктом метаболизма мышечной ткани, возможно ложное занижение или завышение значения СКФ. Примерами могут служить такие клинические ситуации, как беременность, морбидное ожирение или дефицит массы тела, заболевания скелетных мышц, параплегии, квадриплегии и др. В таких ситуациях необходима оценка СКФ клиренсовыми методами (например, проба Реберга–Тареева) [31, 32].
Альбуминурию следует количественно определять посредством соотношения альбумин/креатинин (А/Кр) в разовой порции мочи. Стадия альбуминурии классифицируется как А1 (А/Кр более <30 мг/г), А2 (30–300 мг/г) или А3 (>300 мг/г, включая нефротический синдром >2200 мг/г). В случае выявления экскреции альбумина с мочой это должно быть подтверждено повторным анализом через 3–6 мес. Скрининг на микроальбуминурию следует проводить первично всем пациентам с СД 2, при СД 1 спустя 5 лет после постановки диагноза и далее ежегодно [31, 32].
Помимо вышеперечисленных разделений на основе структурных и функциональных нарушений, существует и морфологическая классификация патологии почек при СД (4 класса), впервые предложенная научным комитетом Общества почечной патологии (США) в 2010 г. и построенная на гломерулярных изменениях [34, 35]:
- класс I: изолированное утолщение базальной мембраны клубочков и лишь легкие неспецифические изменения при световой микроскопии, не соответствующие критериям II–IV классов;
- класс II: мезангиальное расширение (A – легкое, Б – тяжелое) без узлового склероза (изменения Киммелстила–Вильсона) или выраженного гломерулосклероза, затрагивающие более чем 50% клубочков;
- класс III: узловой гломерулосклероз (изменения Киммелстила–Вильсона);
- класс IV: тяжелый диабетический гломерулосклероз (более 50%).
Биопсия почки и морфологическая диагностика могут быть полезны при затруднении определения этиологии ХБП или атипичном течении клинической картины. Показания к биопсии почки включают [32]:
- протеинурию нефротического диапазона при стаже СД менее 5 лет;
- необъяснимую микроскопическую гематурию (особенно акантоцитоз);
- необъяснимое резкое ухудшение функции почек у пациентов с ранее неустановленным диагнозом ХБП;
- снижение СКФ более чем на 30% в течение 2–3 мес у пациентов, получающих лечение ингибиторами АПФ или антагонистами рецепторов ангиотензина II;
- уточнение характера поражения почек при системных заболеваниях.
В силу трудоемкости выполнения биопсии почек этот метод не получил широкого применения в реальной клинической практике. Таким образом, оценка рСКФ и альбуминурии по-прежнему остается краеугольным камнем диагностики и стратификации риска у пациентов с СД и ХБП. При снижении СКФ менее 60 мл/мин/1,73 м2 или двукратном повышении А/Кр более 3 мг/ммоль в интервале более 3 мес выставляется диагноз ХБП.
ХРОНИЧЕСКАЯ БОЛЕЗНЬ ПОЧЕК И САХАРНЫЙ ДИАБЕТ: СТРАТЕГИИ ЛЕЧЕНИЯ
Действующие клинические рекомендации ведущих профессиональных сообществ (American Diabetes Association, KDIGO, Российская ассоциация эндокринологов) рекомендуют многофакторный подход к лечению СД, направленный на коррекцию всех метаболических нарушений, включая уровень гликемии, АД, дислипидемию и массу тела [31, 32, 36]. Такая стратегия была принята на основании полученных результатов крупных исследований за последние десятилетия. Так, в работе DCCT (исследование контроля и осложнений диабета) интенсивный контроль уровня глюкозы уменьшал частоту альбуминурии на 50%, а снижение уровня HbA1c на ≈1% было ассоциировано с меньшим риском микроальбуминурии (на 33%) [37]. Аналогичным образом, по данным масштабных исследований ADVANCE (5 лет наблюдения, n=11 140), ACCORD (n=10 251), UKPDS (10 лет, n=5102), уровень гликемии в пределах целевых значений коррелировал с более низкой частотой повышенной ЭАМ, а снижение систолического АД на 10 мм рт.ст. ассоциировалось с уменьшением риска микрососудистых осложнений, включая нефропатию [38–40]. Также важно отметить, что пациенты с ХБП и СД имеют высокий риск развития потенциально опасных для жизни состояний – гипогликемий. С одной стороны, это связано со снижением синтеза фермента инсулиназы (метаболизирует инсулин) на фоне нефросклероза, а с другой – выведением почками инсулина и сахароснижающих препаратов. Поэтому варианты лечения СД 2 значительно различаются в зависимости от тяжести ХБП.
В соответствии с руководствами American Diabetes Association и Российской ассоциации эндокринологов (2022) выбор сахароснижающих препаратов должен быть персонализированным в зависимости от наличия или отсутствия ССЗ, хронической сердечной недостаточности (ХСН), ожирения и ХБП. Препаратом первой линии для большинства пациентов с СД 2 является метформин [31, 36]. Однако следует помнить: поскольку это лекарственное средство не метаболизируется в печени и выводится почками в неизмененном виде, его следует назначать с осторожностью при СКФ менее 45 мл/мин/1,73 м2 (максимальная доза 1000 мг/сут) и необходимо отменить в случае снижения СКФ менее 30 мл/мин/1,73 м2 [31].
С учетом болезнь-модифицируемого подхода к лечению СД 2 в качестве второй линии терапии этого заболевания стоит рассмотреть применение препаратов, обладающих нефропротективными свойствами: ингибиторов натрий-глюкозного котранспортера 2-го типа (иНГЛТ-2) и/или агонистов рецепторов глюкагоноподобного пептида-1 (арГПП-1) [31, 36].
На сегодняшний день накоплены убедительные данные в отношении кардиоренопротективных свойств иНГЛТ-2. В сентябре 2015 г. были представлены результаты исследования EMPA-REG OUTCOME (эмпаглифлозин), которые впервые продемонстрировали, что терапия эмпаглифлозином у пациентов с СД 2 приводила к снижению риска возникновения ХБП на 39%, макроальбуминурии – на 44%, развития ТСПН у пациентов – на 50% [41]. В свою очередь, в исследовании DECLARE-TIMI 58 применение дапаглифлозина было ассоциировано с 46% снижением риска ухудшения функции почек и с 59% уменьшением вероятности развития ТСПН или смерти от «почечных» причин (ОР 0,41; p=0,012) [42]. Позитивные результаты были получены и в исследовании CANVAS: на фоне терапии канаглифлозином наблюдалось снижение альбуминурии на 27%; снижение достижения конечной почечной точки – на 40% [34].
Принимая во внимание, что вышеперечисленные исследования были нацелены на оценку кардиопротекции и сердечно-сосудистой безопасности иНГЛТ-2, а почечные исходы служили вторичной конечной точкой, особый интерес представляют исследования GREDENCE (канаглифлозин, n=4401 пациент, рСКФ 30–<90 мл/мин/1,73 м2) и DAPA-CKD (дапаглифлозин, n=4304 пациента, рСКФ от 25 до 75 мл/мин/1,73 м2), которые были посвящены именно оценке почечных исходов. Первичная конечная точка в этих исследованиях включала ТСПН (диализ, трансплантация почки или стойкое снижение рСКФ <15 мл/мин/1,73 м2), удвоение показателя креатинина в сыворотке крови или смерть от почечных или сердечно-сосудистых причин. В группе терапии канаглифлозином наблюдалось уменьшение риска развития почечной недостаточности на 30%, кроме того, наблюдалось снижение общего риска неблагоприятного почечного исхода (включая ТСПН, удвоение сывороточных уровней креатинина и смерть от заболеваний почек) на 34%. В рамках исследования DAPA-CKD было показано, что первичная композитная конечная точка встречалась в 9,2% случаев в группе дапаглифлозина и в 14,5% в группе плацебо. Отметим, что в DAPA-CKD исходно диагноз СД имели только 67,5% участников, при этом как в данном исследовании, так и в GREDENCE все пациенты получали терапию блокаторами РААС [43, 44].
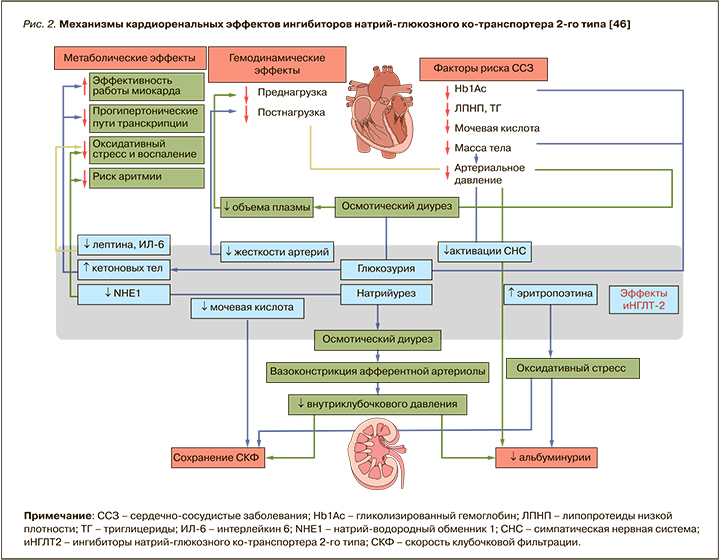
Долгое время существовали опасения относительно применения иНГЛТ-2 у пациентов с продвинутой стадии ХБП ввиду их механизма действия. Препараты этого класса увеличивают концентрацию натрия и хлоридов в области плотного пятна дистального извитого канальца, что усиливает канальцево-гломерулярную обратную связь, вызывая сужение афферентных артериол и снижение внутриклубочкого давления и СКФ [45]. Однако в post-hoc-анализе было установлено, что иНГЛТ-2 проявляли двухфазный эффект в отношении рСКФ, вызывая начальное и обратимое снижение СКФ с последующим долгосрочным сохранением почечной функции. Предикторами резкого снижения СКФ (>10% за 3 нед) являлись пожилой возраст, уровень АД, СКФ >45 мл/мин/1,73 м2, отсутствие ХСН и прием диуретиков в анамнезе [45, 46]. Результаты этих исследований легли в основу изменений инструкций к применению отдельных иНГЛТ-2 у больных СД 2 с продвинутой стадией ХБП. На сегодняшний день дапаглифлозин и канаглифлозин разрешены для лечения пациентов с СД 2 и ХБП 4 стадии, а эмпаглифлозин и ипраглифлозин – при СКФ более 30 мл/мин/1,73 м2. При этом следует помнить, что инициация терапии дапаглифлозином при СКФ менее 25 мл/ мин/1,73 м2 и канаглифлозином при СКФ менее 30 мл/мин/1,73 м2 и уровне альбуминурии >300 мг/сут противопоказана, однако возможно продолжить такую терапию у ранее получавших ее пациентов с целью уменьшения риска наступления ТСПН, смерти от ССЗ и госпитализации по поводу ХСН [36]. Резюмируя все вышесказанное, можно констатировать, что иНГЛТ-2 обладают не только выраженным нефропротективным эффектом, но и хорошим профилем безопасности (рис. 2).
При наличии противопоказаний к назначению иНГЛТ-2 (например, инфекции мочевыводящих путей) или недостижении целей гликемического контроля комбинацией метформин + иНГЛТ2 предпочтение следует отдавать группе препаратов арГПП-1 [36].
Нефропротективные свойства арГПП-1 обусловлены не только их гипогликемическим эффектом, но и множественными плейотропными механизмами, включая снижение веса, АД, противовоспалительным и антиоксидантным действием. Так, крупнейший метаанализ (n=56 004) исследований EXSCEL (эксенатид пролонгированного действия) [47], ELIXA (ликсисенатид) [48], LEADER (лираглутид) [49], SUSTAIN-6 (семаглутид) [50], REWIND (дулаглутид) [51], PIONEER 6 (пероральный семаглутид) [52] и Harmony Outcome (албиглутид) [53] продемонстрировал снижение риска комбинированного почечного исхода на 17% (ОР 0,83; 95% ДИ: 0,78–0,89) главным образом за счет макроальбуминурии [54]. Правда, важно подчеркнуть, что все вышеперечисленные исследования арГПП-1 были направлены на оценку сердечно-сосудистой безопасности, тогда как ренальные исходы рассматривались в качестве вторичных конечных точек.
Первым исследованием, изучающим влияние арГПП-1 на прогрессирование почечной дисфункции у пациентов с СД 2, стало исследование FLOW (семаглутид), результаты которого ожидаются в 2024 г.
В целом терапию арГПП-1 у пациентов с СД 2 и ХБП следует рассмотреть при невозможности назначения иНГЛТ-2 или при недостижении индивидуальных целей гликемического контроля на фоне комбинированной терапии другими сахароснижающими средствами. Причем препараты этой группы могут быть рекомендованы пациентам с тяжелым нарушением функции почек: экзенатид и ликсисенатид при рСКФ более 30 мл/ мин/1,73 м2, лираглутид, дулаглутид, семаглутид – при рСКФ >15 мл/мин/1,73 м2.
У пациентов с более выраженным нарушением почечной функции или при невозможности назначения препаратов выбора следует рассмотреть терапию ингибиторами дипептидилпептидазы 4 (иДПП-4). В то же время, учитывая, что иДПП4 выводятся и метаболизуются по-разному, может потребоваться коррекция/снижение их дозы в зависимости от уровня СКФ. Линаглиптин и гемиглиптин – два представителя этого класса препаратов, которые не выводятся почками и, следовательно, могут использоваться на всех стадиях ХБП без коррекции дозы [31]. Интересно, что в ретроспективном исследовании Chen J.-J. et al. (n=27 279) было продемонстрировано, что терапия арГПП-1 у пациентов с СД 2 и ТСПН ассоциировалась с меньшим числом смертельных исходов в сравнении с иДПП-4 [55]. Для подтверждения преимущества назначения арГПП- 1 у пациентов с ТСПН требуется проведение хорошо спланированных рандомизированных исследований.
Беря в расчет высокий риск гипогликемических состояний у пациентов с ХБП, инсулины и средства, стимулирующие секрецию инсулина (препараты сульфонилмочевины, глиниды), следует применять с осторожностью по мере прогрессирования заболевания почек. Так, глибенкламид противопоказан пациентам с СД 2 при рСКФ менее 60 мл/ мин/1,73 м2 в связи с высоким риском тяжелых гипогликемий: он метаболизируется в печени, но его активные метаболиты экскретируются с мочой. Гликлазид, глимепирид и глипизид могут быть назначены пациентам с нарушением функции почек (до ХБП С4 стадии), поскольку они метаболизируются в печени и выводятся с мочой в виде неактивных метаболитов. Вместе с тем их применение должно проходить под тщательным контролем [56].
Таким образом, у пациентов с СД 2 и ХБП в качестве первой линии терапии следует назначать комбинацию метформин + иНГЛТ2 и/или арГПП- 1. Применение иДПП-4 может быть рассмотрено при СД 2 на любой стадии ХБП, а том числе и при СКФ менее 15 мл/мин/1,73 м2 с соответствующим снижением дозы, за исключением линаглиптина и гемиглиптина. Ведение пациентов, получающих инсулинотерапию и препараты сульфонилмочевины, должно осуществляться под тщательным контролем ввиду высокого риска гипогликемических реакций.
ЗАКЛЮЧЕНИЕ
ХБП является частым осложнением СД и связана с неблагоприятными клиническими исходами, включая повышенный риск смертности от всех причин и ССЗ, а также с неблагоприятными экономическими и социальными последствиями. В течение последних десятилетий отмечается гетерогенность течения ДН. В частности, были описаны два ее новых фенотипа – неальбуминурическая и альбуминурическая. Раннее выявление как СД, так и ХБП имеет решающее значение для снижения осложнений, заболеваемости и смертности у этих больных. Диагностика и скрининг ХБП заключаются в определении А/Кр в разовой порции мочи и расчете СКФ. В случае выявления нарушения функции почек пациенты должны получать препараты, обладающие нефропротективными свойствами (иНГЛТ-2 и/или арГПП-1), а также адекватную терапию, направленную на коррекцию факторов риска ССЗ.



