ВВЕДЕНИЕ
Колонизация Helicobacter pylori (H. pylori) поверхности и складок слизистой оболочки желудка значительно затрудняет антибактериальную терапию. Успешная схема лечения основана на сочетании препаратов, которые предотвращают возникновение резистентности и настигают бактерию в различных участках желудка. Терапия должна гарантировать, что даже небольшая популяция микроорганизмов не останется жизнеспособной.
Эрадикационная терапия H. pylori представляет собой использование комбинации двух антибактериальных средств, препарата висмута и ингибитора протонной помпы в течение 14 дней. Несмотря на усиленную терапию, частота эффективной эрадикации неуклонно снижается из-за усиления антибиотикорезистентности H. Pylori, что требует разработки «новых» антибиотиков. Показательный факт: еще в 2017 г. H. pylori, резистентный к кларитромицину, был включен Всемирной организацией здравоохранения в список 12 приоритетных возбудителей [1].
Известно, что создание новых антибактериальных средств, подходящих для эрадикации H. pylori, сопряжено с рядом трудностей. Основная проблема – высокая стоимость разработки и вывода на рынок нового препарата [2]. Усугубляет положение и то обстоятельство, что даже после успешной регистрации антибактериального препарата регуляторные органы, как правило, в целях предотвращения быстрого развития резистентности ограничивают обращение таких препаратов [3]. Вследствие этого крупные фармацевтические компании все чаще отказываются от исследований в области разработки новых антибиотиков.
В связи с вышесказанным представляется перспективной разработка структурно новых препаратов, представляющих собой уже одобренные антибактериальные средства, с использованием нанотехнологий [4].
Под термином «нанотехнологии» понимают совокупность методов и приемов, обеспечивающих возможность контролируемым образом создавать и модифицировать объекты, включающие компоненты с размерами менее 100 нм, имеющие принципиально новые качества и позволяющие осуществлять их интеграцию в полноценно функционирующие системы большего масштаба. Применение нанотехнологий является определенной альтернативой для улучшения и усиления эффекта существующих противомикробных препаратов.
Цель данной статьи – обзор результатов исследований в области нанотехнологий для оптимизации лечения H. Pylori-ассоциированных заболеваний и оценка дальнейших перспектив развития в области нанофармакологии.
ОСНОВНЫЕ МЕХАНИЗМЫ ДЕЙСТВИЯ НАНОЧАСТИЦ
Одним из главных направлений нанотехнологии в лечении H. pylori является создание наночастиц, содержащих в своем составе молекулы антибактериальных средств. Первоначально основная задача таких наночастиц заключалась в защите лекарственных средств от агрессивной кислой среды желудка или повышении растворимости препарата [5, 6]. Позже исследователи обнаружили, что нанопереносчики, «нагруженные» антибиотиком, используют одновременно несколько механизмов для подавления роста или уничтожения H. pylori, что затрудняет развитие у бактерий лекарственной устойчивости.
Некоторые наночастицы обладают способностью проникать через барьеры слизистой оболочки или даже пенетрировать во внеклеточный слой матрикса микробных биопленок и вызывать его деградацию [7, 8]. Системы доставки лекарств на основе наночастиц могут взаимодействовать с бактериальной клеткой двумя разными механизмами [9]. При реализации первого механизма наночастица сливается (соединяется) с клеточной стенкой или клеточной мембраной микроорганизма и транспортирует молекулы антибиотика через них. С помощью другого механизма наночастицы адсорбируют отрицательно заряженные бактерии. Адсорбция увеличивает проницаемость мембраны, лекарственное средство непрерывно высвобождается на клеточной стенке и диффундирует внутрь бактериальной клетки [5, 9].
Таргетная доставка антибактериальных средств и их пролонгированное высвобождение нанопереносчиками позволяют достичь эффективной концентрации лекарственного препарата в бактериальных клетках-мишенях, что дает пероральным наноантибиотикам уникальные преимущества в плане уничтожения резистентных штаммов H. pylori. Кроме того, было обнаружено, что по сравнению с традиционными антибиотиками наночастичные системы менее токсичны при лечении [10].
Выделяют несколько категорий нанопереносчиков с антибиотиками, предназначенных для эрадикации H. pylori:
- наночастицы на основе липидов, такие как липосомы, твердые липидные наночастицы, наноструктурированные липидные носители и наноэмульсии (рис. 1);
- полимерные наночастицы – полимолочная и полигликолевая кислоты, полиэтиленгликоль, хитозан;
- неорганические наночастицы, такие как металлические наночастицы.
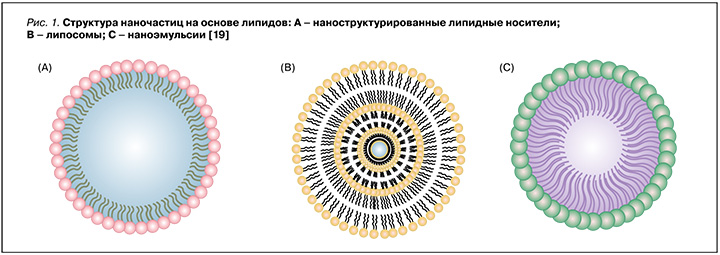
Существуют и другие нанопереносчики, причем каждый из них демонстрирует отличительные преимущества в доставке антимикробных препаратов.
В ряде научных работ сообщалось, что, помимо инкапсулирования антимикробных средств, некоторые нанопереносчики на основе липидов, наночастицы хитозана и неорганические наночастицы (такие как наночастицы серебра) обладают собственной антимикробной активностью [11–13]. Существуют также гибридные наночастицы – стабилизированные липосомы, сочетающие достоинства различных типов наноносителей, таких как липидно-полимерные гибридные наночастицы и металлические наночастицы [14, 15]. Наряду с этим была разработана серия биомиметических наночастиц с использованием биомембран клеток (таких как клетки крови человека или животного, иногда даже бактериальные клетки) для изготовления нанопереносчиков, которые можно было бы использовать для вакцинации [16–18].
Использование наноносителей способствовало разнообразию доставки противомикробных средств, применяемых для эрадикации H. pylori.
ЛИПОСОМАЛЬНЫЕ ПРЕПАРАТЫ ПРОТИВ H. PYLORI
Одними из наиболее изученных и успешно применяемых средств адресной доставки лекарственных препаратов являются липосомы. Липосомы – это полые самообразующиеся частицы сферической формы, размером от 50 до 1000 нм [20]. Водное ядро липосом окружено одним или несколькими фосфолипидными бислоями. Такие частицы, с одной стороны, могут инкапсулировать как липофильные лекарственные препараты в билипидный слой мембраны, а с другой – как гидрофильные препараты – в водное ядро липосом. Они также служат универсальными носителями лекарств, поскольку физико-химические свойства липосом могут быть легко преобразованы путем изменения фосфолипидов, их доли и размера, заряда и даже чувствительности к внешним воздействиям, таким как рН и температура [21]. Кроме того, их сходство с клеточной мембраной позволяет осуществлять слияние с бактериями путем эндоцитоза [22]. В случае инфекции H. pylori фосфолипиды могут создавать гидрофобный слой, способный препятствовать прикреплению бактерий к слизистой оболочке и поставлять жирные кислоты для восстановления слизистой оболочки желудка [19]. В тоже времялипосомы, какправило, непригодны дляпероральноговведения, посколькунестабильны при хранении и в биологической среде [23, 24]. Исследователи Jain P. et al. включили метронидазол и амоксициллин в липосомы и покрыли их полиакриловой кислотой и полиаллиламина гидрохлоридом для стабилизации структуры [25]. Преимущество такой многослойной структуры в том, что наличие двух антибактериальных препаратов в одной везикуле позволяет преодолевать резистентность H. pylori и повышает эффективность эрадикации, а полимерное покрытие обеспечивает прочную гидрофобную оболочку для защиты ядра липосомы от деградации в кислой среде желудка. Это было отражено в исследовании in vitro: кумулятивное высвобождение лекарственного средства из липосом с полимерным покрытием в среде, имитирующей желудочный сок (pH 1,2), составило 52–55% в течение 24 ч, тогда как простые липосомы высвобождали более 95% инкапсулированного лекарственного средства в течение 6 ч. При использовании липосомы с полимерным покрытием, загруженной лекарственным средством, эффективность эрадикации у мышей, инфицированных H. pylori, была выше, по сравнению с введением простых липосом с инкапсулированными антибиотиками. Эти исследования показали, что липосомы сами по себе могут быть недостаточно эффективными для доставки антибиотиков в желудок, поэтому интеграция полимеров может быть эффективнее при эрадикации H. pylori [25].
В ряде клинических исследований свободные жирные кислоты, такие как стеариновая, олеиновая и линолевая кислоты, были инкапсулированы в липосомы [26]. Было установлено, что жирные кислоты обладают как бактерицидной, так и бактериостатической активностью в отношении широкого спектра бактерий [27]. Так, несколько исследований продемонстрировали, что H. pylori чувствителен к жирным кислотам, включая линоленовую кислоту in vitro. Однако степень антихеликобактерного эффекта жирных кислот остается до конца неясной, поскольку большинство свободных жирных кислот, эффективных против H. pylori, плохо растворимо и нестабильно [28].
В исследовании 2015 г. Jung S.W. et al. оценивали эффективность липосомальной липоленовой кислоты (ЛЛК) по сравнению с липосомальной стеариновой (ЛСК) и липосомальной олеиновой (ЛОК) кислотами в отношении нескольких изолированных резистентных штаммов H. pylori, включая как спиральную, так и кокковидную их форму. ЛЛК показала наиболее мощный бактерицидный эффект, полностью уничтожив H. pylori в течение 5 мин. Проницаемость наружной мембраны H. pylori повышалась при лечении ЛЛК и ЛОК. Помимо этого, путем детекции высвобождаемого АТФ из бактерий было обнаружено, что плазматические мембраны H. pylori, обработанные ЛЛК, имели значительно более высокую проницаемость, чем те, которые обрабатывались ЛОК; это влекло за собой гибель бактериальных клеток. Просвечивающая электронная микроскопия и сканирующая электронная микроскопия (рис. 2) позволили установить, что ЛЛК вызывала структурные изменения в бактериальной мембране в течение 5 мин, что приводило к высвобождению внутриклеточных компонентов, таких как АТФ [26].
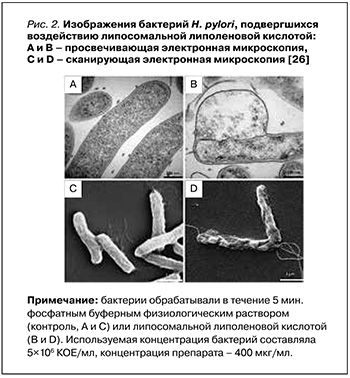
Кроме того, та же исследовательская группа обнаружила, что ЛЛК проникает в слизистый слой желудка мыши и сохраняется в ней. ЛЛК обладала превосходной антибактериальной эффективностью in vivo по сравнению со стандартной тройной антихеликобактерной терапией в желудке мыши [29].
Таким образом, основываясь на сильном и быстром бактерицидном действии ЛЛК, а также уже описанном действии этого вещества in vivo, предполагается, что ее применение может служить потенциально новой и перспективной терапевтической стратегией в лечении H. pylori.
ПОЛИМЕРНЫЕ НАНОЧАСТИЦЫ
Наноносители на полимерной основе обладают очень хорошей стабильностью и нагрузочной способностью, что определяет их эффективную таргетную доставку. Типичными соединениями, которые представляют основу для создания полимерных наночастиц, являются полимолочная и полигликолевая кислоты, полиэтиленгликоль, хитозан [30]. В отличие большинства биополимеров хитозан имеет реакционноспособную группу (гидроксильную и/или аминогруппу) в каждом звене, что позволяет ему присоединять несколько молекул, необходимых для функционирования частиц [31].
Хитозан – природный линейный поликатионный гетерополисахарид с высокой молекулярной массой. Он состоит из D-глюкозаминовых и N-ацетилD-глюкозаминовых звеньев, не растворимых при нейтральном рН. Чаще всего хитозан извлекают из панцирей креветок и крабов, также он содержится в кутикулах насекомых [32]. Этот полисахарид характеризуется низкой токсичностью, при этом быстро ионизируется и растворяется в кислой среде желудка. Хитозан и его производные обладают мукоадгезивными свойствами, т.е. способны адгезировать к слизистой оболочке желудка благодаря электростатическому взаимодействию между положительно заряженным полимером, отрицательно заряженными гликопротеинами слизистой оболочки желудка и слизью, что продлевает время их пребывания в желудке [33]. С другой стороны, положительно заряженные NH2-группы хитозана взаимодействуют с отрицательно заряженными группами, расположенными на поверхности бактерий, повышая проницаемость клеточных мембран и вызывая в некоторых случаях гибель бактериальных клеток [34]. Кроме того, хитозан обладает подавляющим влиянием на кворум сенсинг (quorum sensing, QS) – сигнальный механизм, с помощью которого микробные колонии в биопленке контролируют экспрессию генов в ответ на непрерывные изменения плотности клеточной популяции. Поскольку QS играет ключевую роль в выживании бактерий, влияние хитозана приводит к уничтожению биопленки. Эффективность разрушения биопленок в данном случае обусловлена нарушением передачи сигнальных молекул для межклеточной коммуникации [35].
Эффективность инкапсуляции амоксициллина в наночастицы хитозана для лечения H. pylori была продемонстрирована in vivo с использованием крыс линии Вистар [36]. Исследование проводилось путем перорального введения порошка амоксициллина либо мукоадгезивных микрочастиц в однократной или многократной дозе. Согласно полученным результатам, мукоадгезивные микрочастицы амоксициллина обладают лучшим бактерицидным эффектом по сравнению с порошком амоксициллина. Эффективность мукоадгезивных микрочастиц определяется замедленным высвобождением амоксициллина в желудочно-кишечном тракте (ЖКТ) и повышенной стабильностью антибиотика в кислой среде желудка.
Для предотвращения преждевременного высвобождения инкапсулированного лекарственного средства молекулы хитозана конъюгируют с лектинами. Исследователи Jain S.K. et al. разработали систему доставки лекарственных средств путем конъюгации лектина (конканавалин A, Con-A) с микросферами хитозана, нагруженными кларитромицином. Эффективность разработанного препарата в ЖКТ была визуализирована с помощью гамма-сцинтиграфии у новозеландских кроликов-альбиносов. У всех подопытных животных длительность адгезии микросфер к слизистой оболочке желудка превышала 6 ч [37].
Исследователи под руководством Ramteke S. изучали воздействие наночастиц с фукозой на лектиновые рецепторы С-типа на поверхности H. pylori. Конъюгированные с фукозой наночастицы, содержащие амоксициллин/кларитромицин/омепразол (компоненты тройной антихеликобактерной терапии), были получены путем ионотропного гелеобразования. Процент захвата лекарственного средства и профили высвобождения указанных лекарственных средств в моделируемой желудочной жидкости (рН 1,2) определяли с помощью высокоэффективной жидкостной хроматографии. In vitro при воздействии конъюгированными с фукозой наночастицами частота эрадикации H. pylori составила 97%, в то время как при использовании неконъюгированных наночастиц хитозана – 91%, а при применении стандартной тройной терапии – только 81%. Результаты исследования in vivo на H. pylori-инфицированных швейцарских мышах-альбиносах продемонстрировали, что конъюгированные с фукозой наночастицы, содержащие компоненты тройной антихеликобактерной терапии (53,57 мг/кг амоксициллина, 17,8 мг/кг кларитромицина и 1,42 мг/кг омепразола), способны полностью уничтожить хеликобактерную инфекцию [38].
МЕТАЛЛИЧЕСКИЕ НАНОНОСИТЕЛИ
Проведен ряд доклинических исследований, подтверждающих, что металлические наночастицы, такие как серебро, золото, висмут, цинк и железо, обладают бактерицидным действием в отношении H. pylori [33, 39]. Основные механизмы действия металлических наночастиц реализуются через оксидативный стресс, высвобождение ионов металлов и неокислительный стресс [40].
Клинический и научный интерес представляют наночастицы серебра, синтезированные с использованием экстрактов различных растений. Концентрация растительного экстракта и субстрата, температура, реакции и рН значительно влияют на размер, форму и стабильность наночастиц серебра. Исследователи под руководством Gurunathan S. синтезировали наночастицы серебра с использованием экстракта листьев японской полыни (Artemisia princeps) [41]. Основные результаты этого исследования продемонстрировали дозозависимое снижение жизнеспособности клеток и образования биопленок H. pylori, а также усиленное образование активных форм кислорода и фрагментацию ДНК бактериальных клеток.
Согласно исследованию Mikhailova E.O., механизм действия наночастиц серебра на клетки включает несколько этапов: адгезию на поверхности бактериальной клеточной стенки, проникновение в клетку и разрушение внутриклеточных органелл и биомолекул, индукцию окислительного стресса и модуляцию сигнальных путей [42]. Общепризнано, что бактерицидные свойства наночастиц серебра связаны с нарушением транскрипции РНК, пуринов, пиримидинов и жирных кислот бактерий, а также взаимодействием наночастиц с клеточной ДНК и нарушением ее репликации.
Saravanan M.С. et al. синтезировали анизотропные структурированные наночастицы оксида цинка с применением бесклеточного экстракта бактерий Bacillus megaterium. Кроме того, в этом исследовании изучалось действие наночастиц оксида цинка на штаммы H. pylori и оценивалась их безопасность в нормальных мезенхимальных стволовых клетках человека. Наночастицы оксида цинка продемонстрировали высокую биосовместимость с мезенхимальными стволовыми клетками человека и оказались потенциально безопасными в клетках млекопитающих. Исследования антибактериальной активности данной лекарственной формы показали значительный ее бактерицидный эффект в отношении штаммов H. pylori с множественной лекарственной устойчивостью [43].
Соединения висмута, в основном висмута трикалия дицитрат, уже давно используются в стандартной антихеликобактерной терапии первой линии. В 2006 г. были синтезированы нанотрубки субкарбоната висмута ((BiO)2CO3) из цитрата висмута и мочевины в этиленгликоле. Chen R. et al. сравнивали антибактериальную активность нанотрубок (BiO)2CO3 и наночастиц оксида висмута (Bi2O3) с коллоидным субцитратом висмута, применяя метод серийного разведения на агаре в отношении клинических изолятов H. pylori. В соответствии с полученными результатами доклинических исследований нанотрубки (BiO)2CO3 проявляют антибактериальные свойства (МIC50 составляет 10 мкг/мл-1) и превосходят в ингибирующем эффекте клинически используемый коллоидный субцитрат висмута, однако наночастицы Bi2O3 почти не обладают бактерицидным действием в отношении H. pylori. Ожидается, что нанотрубки могут быть использованы в качестве капсулы для других лекарственных средств из-за их уникальной наноструктуры, собственной антибактериальной активности и способности растворяться в кислой среде желудка [44].
ЗАКЛЮЧЕНИЕ
Повышение эффективности эрадикационной терапии H. pylori продолжается в разных направлениях, среди которых наиболее перспективным представляется использование наноматериалов в клинической практике. Однако практическое применение нанопрепаратов сопряжено с рядом проблем, включая невысокую стабильность наночастиц, их деградацию в ЖКТ и взаимодействие с клетками иммунной системы, что требует всесторонней оценки их биосовместимости. Некоторые наноматериалы не поддаются биологическому разложению в организме человека, и их влияние на здоровые клетки не изучено. Например, металлические наночастицы могут оставаться в организме после использования в течение длительного времени и усиливать цитотоксичность. Также важно отметить, что в современной медицине не разработаны единые стандарты для нанопрепаратов [45].
Тем не менее проведенные исследования наглядно свидетельствуют, что нанотехнологии позволяют применять новые лекарственные формы с улучшенными фармакокинетическими показателями уже известных антибактериальных средств. Ожидается, что в ближайшее время будет разработано и одобрено для клинических испытаний больше наноматериалов. В будущем нанопрепараты, вероятно, станут более широко использоваться в клинической практике и обеспечат высокую эффективность эрадикации даже у пациентов с резистентными штаммами H. pylori.



