Успехи современной медицины приводят к увеличению количества пациенток детородного возраста, перенесших кардиохирургические вмешательства, включая женщин с протезами клапанов сердца (ПКС), которым ранее из-за высоких рисков осложнений, в том числе материнской летальности, планирование беременности было противопоказано. Вопрос выбора тех или иных ПКС у женщин детородного возраста до сих пор остается нерешенным. При необходимости протезирования клапанов сердца предпочтение отдается механическим протезам ввиду их практически неограниченного срока службы; в этом их принципиальное отличие от биологических протезов, чей срок службы ограничен и требует в определенный момент репротезирования [1].
В то же время наличие механического протеза, в отличие от биологического, требует пожизненного приема антикоагулянтной терапии. Кроме того, наличие механического протеза клапана (МПК) сопряжено с рядом осложнений: тромбоэмболическими осложнениями, инфекционным эндокардитом, кровотечениями, нарушениями ритма сердца, сердечной недостаточностью, дисфункцией протеза.
Согласно классификации Всемирной организации здравоохранения (ВОЗ) наличие МПК у беременной относится к риску III, что означает значительное увеличение вероятности материнской смертности или развития тяжелых осложнений. Наблюдение этой категории пациенток включает не только оценку рисков материнской летальности и осложнений, но и требует выбора стратегии антикоагулянтной терапии. Каждый из существующих в настоящее время режимов антикоагулянтной терапии оказывает значимое влияние на исходы беременности как со стороны матери, так и со стороны плода.
У каждого варианта антикоагулянтной терапии есть свои плюсы и минусы. К сожалению, существующие на сегодняшний момент рекомендации по выбору антикоагулянтного режима и тактике ведения этой категории пациенток основываются на доказательствах низкого уровня: в основном это результаты небольших ретроспективных исследований, либо небольших регистров [2, 3]. Ведение беременности у пациенток c ПКС осложняется гемодинамическими нарушениями и физиологической гиперкоагуляцией, которая служит дополнительным риском тромбоэмболических осложнений [2]. Кроме того, наличие МПК повышает риск кровотечений в случае экстренного родоразрешения у пациенток, принимающих антикоагулянты.
У женщин с биологическим протезом клапана (БПК) исключен фактор влияния антикоагулянтной терапии на исходы беременности и плода. Однако, несмотря на отсутствие необходимости в приеме антикоагулянтов, у пациенток с БПК в сравнении с общей популяцией по результатам предыдущих исследований также регистрировалась более высокая частота как геморрагических, так и тромбоэмболических осложнений [4].
В настоящее время исходы беременности во многом определяются опытом и возможностями медицинского центра, в котором наблюдается и родоразрешается пациентка. Целью настоящей работы стал анализ частоты и выявление факторов риска тромботических и геморрагических осложнений во время беременности, родов и послеродового периода у женщин с ПКС, которые наблюдались в условиях перинатального центра, работающего в составе многопрофильного стационара.
МАТЕРИАЛЫ И МЕТОДЫ
За период с января 2011 г. по декабрь 2018 г. в условиях специализированного перинатального центра ФГБУ «НМИЦ им. В.А. Алмазова» (Санкт-Петербург) родоразрешена 20 831 пациентка, у 9137 (43,9%) из них выявлены различные заболевания сердечно-сосудистой системы. За указанный период в условиях перинатального центра во время беременности наблюдались 44 пациентки с ПКС, 7 из них родоразрешены дважды. Таким образом, в ретроспективное когортное исследование был включен 51 случай беременности у пациенток с ПКС.
В зависимости от типа клапанного протеза пациентки были разделены на 2 группы: в первую были включены 34 случая беременности у 30 пациенток с МПК, во вторую – 17 случаев беременностей у 14 пациенток с БПК.
Исследование выполнено в соответствии со стандартами надлежащей клинической практики (Good Clinical Practice) и принципами Хельсинской декларации. У всех пациенток перед включением в исследование учитывалось наличие информированного согласия на обработку персональных данных, одобренное этическим комитетом ФГБУ «НМИЦ им. В.А. Алмазова» Минздрава России.
Всем пациенткам проводилось трансторакальное эхокардиографическое исследование (ЭхоКГ) на аппарате Vivid 7 GE по стандартному протоколу, согласно рекомендациям [5, 6], не менее 3 раз в каждом триместре беременности. При подозрении на дисфункцию, тромбоз протеза выполнялось чреспищеводное ЭхоКГ на любом сроке беременности.
В качестве критерия патологической кровопотери в родах рассматривался объем более 1 л после оперативного родоразрешения и более 0,5% от массы тела пациентки в случае естественных родов.
Статистический анализ данных, полученных в ходе исследования, проведен с использованием прикладных статистических программ Statistica for Windows ver. 10.0. Поскольку большинство количественных показателей имело распределение, отличающееся от нормального, для сравнения подгрупп использовался критерий Манна–Уитни, а для описания подгрупп – медианы и квартили. Для сравнения подгрупп по бинарным показателям применялся точный критерий Фишера. Критерий значимости устанавливался на уровне р <0,05.
РЕЗУЛЬТАТЫ
Средний возраст наблюдавшихся беременных женщин составил 30,3±5,2 лет (от 13 до 42 лет).
В 66,7% случаев (n=34) у беременных пациенток произведена в анамнезе имплантация МПК, в 33,3% (n=17) – БПК. Пациентки с биологическими протезами были значимо старше (табл.).
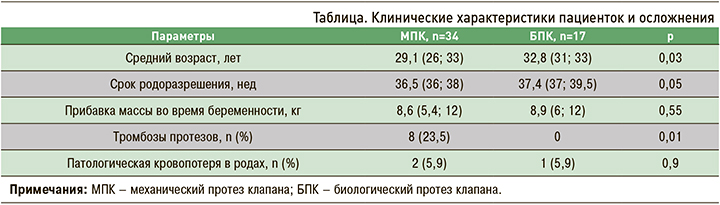
По сроку родоразрешения и прибавке в весе группы были сопоставимы. Наиболее частой этио-логической причиной протезирования были различные варианты врожденных пороков сердца, чаще всего двустворчатый аортальный клапан и полный/неполный атриовентрикулярный канал. Не менее часто причиной протезирования становился первичный и вторичный инфекционный эндокардит (рис. 1).
Среди основных гемодинамических причин протезирования фигурировали тяжелая митральная недостаточность и тяжелый аортальный стеноз (рис. 2).
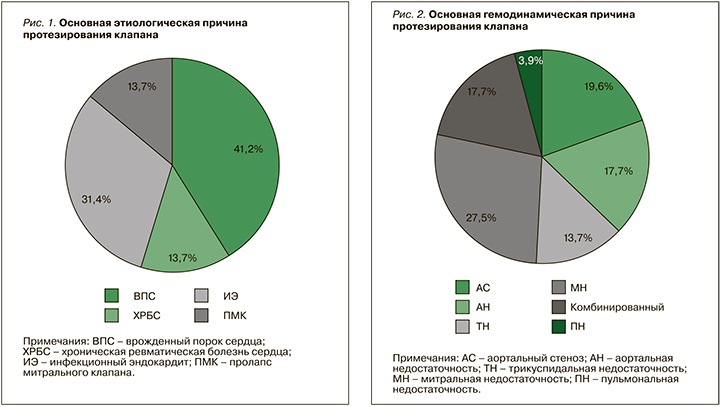
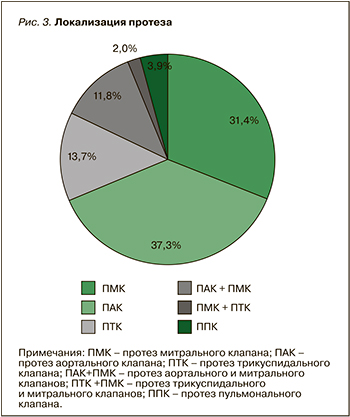
Этим фактом объясняется более частая встречаемость протезов митрального клапана (31,4%) и аортального клапана (37,3%), что отражено на рисунке 3. Из 6 пациенток с тяжелой трикуспидальной недостаточностью в 5 случаях был имплантирован биологический протез.
 В группах наблюдения беременность завершилась рождением 46 жизнеспособных детей.
В группах наблюдения беременность завершилась рождением 46 жизнеспособных детей.
У 5 пациенток зарегистрирована остановка развития плода в I и II триместрах: 3 случая неразвивающейся беременности (8,8%) на сроках 7, 10 и 20 нед у пациенток с МПК, которые получили терапию варфарином. В группе пациенток с БПК выявлено 2 случая (11,8%) остановки развития плода: 1 случай (5,9%) – выкидыш раннего срока (10 нед) и 1 (5,9%) – антенатальная гибель плода на сроке 24 нед. Таким образом, существенных различий по исходам беременности у пациенток МПК, несмотря на прием антикоагулянтной терапии, по сравнению с пациентками с БПК, не выявлено. Только в одном случае установлена причина неразвивающейся беременности как следствие тератогенного эффекта варфарина.
Анализ анамнестических данных показал, что указания на неразвивающиеся беременности были у 5 пациенток с БПК (36%) и практически у каждой третьей пациентки с МПК (30%). Следует подчеркнуть, что только у 3 пациенток с БПК и у 12 с МПК беременность, которую вели специалисты перинатального центра, была первой.
В группе женщин с МПК у каждой четвертой пациентки (26,4%) регистрировались тромботические осложнения во время беременности (n=9), из них 8 (23,5%) случаев тромбозов протеза и 1 случай острого нарушения мозгового кровообращения. Наиболее часто тромбозы регистрировались при наличии МПК в митральной позиции – 33%, тогда как в аортальной позиции только у 18%. Кроме того, тромбоз протеза верифицирован у единственной пациентки с МПК в трикуспидальной позиции и единственной пациентки с двумя протезами в митральной и трикуспидальной позиции. В 3 случаях тромбозы протезов были связаны с самостоятельной отменой пациентками антикоагулятной терапии (33%), в одном случае с несоблюдением режима назначения низкомолекулярных гепаринов (НМГ). Несмотря на тромбозы, лишь в 2 случаях из 8 потребовалось репротезирование во время беременности и в одном случае после родов. У 6 женщин функция протезов восстанавливалась после назначения оптимальной антикоагулянтной терапии, и повторного оперативного вмешательства не потребовалось. В послеродовом периоде не было зарегистрировано ни одного нового случая тромбоза протеза.
В группе пациенток с БПК в 10 случаях (58,8%) родоразрешение было выполнено оперативным путем, причем в 8 случаях по акушерским показаниям. Таким образом, в этой группе в 41,2% (n=7) случаев родоразрешение произошло через естественные родовые пути, тогда как в группе с МПК аналогичный показатель составил лишь 5,9% (92 случая) (р=0,02). Однако частота экстренных оперативных вмешательств у обследованных пациенток первой группы была значительно меньше (р=0,03). При этом пациентки в изучаемых группах не отличались по частоте патологической кровопотери в родах независимо от способа родоразрешения. В раннем послеродовом периоде кровотечения с формированием подапоневротической гематомы, потребовавшие релапаратомии и эвакуации гематомы, наблюдались у 5 пациенток с МПК (14,7%). Терапия варфарином у 4 из них была возобновлена в первые сутки после родоразрешения и лишь в одном случае на вторые сутки. Несмотря на случившиеся тромботические и геморрагические осложнения, за время наблюдения не зарегистрирован не один случай материнской летальности и не потребовалась органоуносящая хирургическая остановка кровотечения.
ОБСУЖДЕНИЕ
С каждым годом увеличивается количество пациенток детородного возраста с протезированными клапанами, планирующих беременность [7, 8]. Однако, несмотря на существующие рекомендации по ведению беременных пациенток с ПКС, материнская летальность у этой группы пациенток и частота других серьезных осложнений как со стороны матери, так и со стороны плода остаются довольно высокими.
Наименьшая летальность зарегистрирована по результатам наблюдательного регистра беременных женщин с кардиоваскулярной патологией (ROPAC). Среди включенных в ROPAC 212 женщин с МПК и 134 с БПК материнская летальность составила 1,4% в первой категории пациенток и 1,5% во второй [9].
Врожденные пороки сердца по результатам настоящего исследования были основной причиной протезирования клапанов, аналогичные результаты получены и в Национальном регистре Дании. Сопоставима была и локализация протеза клапана – частота протезирования аортального клапана в нашем исследовании составила 37,3%, митрального клапана – 31,4%, тогда как в регистре Дании аналогичные показатели составили 42 и 25% соответственно [8]. При этом тромбозы протезов, как и в нашем исследовании, чаще регистрировались у пациенток с протезом митрального клапана. Напротив, по данным регистра ROPAC, частота тромбозов у пациенток с протезом митрального клапана не превышала 4,4%, а у пациенток с протезом аортального клапана – 2,6% [9]. Согласно результатам Vause S. et al., тромбозы протезов регистрировались только у женщин с МПК митрального клапана. У этой же категории у пациенток регистрировалась наибольшая частота различных осложнений – 57%, в то время как у пациенток с протезированием аортального клапана они наблюдались только в 39% случаев [10].
В настоящем исследовании отмечена высокая частота тромбозов – у 23,5% пациенток с МПК; это превышает максимальные показатели метаанализа, проведенного D’Souza R. et al., в котором частота тромбоэмболических осложнений у пациенток с МПК при беременности колебалась от 1,4 до 19,6% [11]. В то же время полученный в нашем исследовании результат сопоставим с данными анализа Popelova J. et al., в котором частота тромбоза протеза составила 26% [12]. Наименьшая частота тромбоэмболических осложнений была зарегистрирована в исследовании Martin S. et al.: лишь у одной пациентки (0,9%) из 107 беременных с МПК (период анализа с 1997 по 2007 г.) [8]. Только в 5% случаев были документированы тромбозы МПК в регистре ROPAC.
Высокая частота тромбозов протезов, наблюдавшаяся в нашем исследовании, обусловлена в первую очередь низкой комплаентностью и самостоятельной отменой антикоагулянтной терапии пациентками. Второй немаловажной причиной тромботических осложнений была субтерапевтическая дозировка назначаемых НМГ. Большинство других исследований, описывающих случаи тромбоза у пациенток, получающих НМГ, также отмечали их возникновение либо при отсутствии контроля анти-Ха активности, либо на субтерапевтическом уровне анти-Ха активности [13–18].
Согласно мировым исследованиям, применение высокой дозировки варфарина [8, 9] влечет за собой развитие эмбриопатии (8% в I триместре), антенатальную гибель плода, риск преждевременных родов, кровотечения при ургентной акушерской ситуации. В настоящем исследовании эмбриозпатия зарегистрирована лишь у одной пациентки, получавшей 5 мг варфарина в I триместре. Однако антенатальная гибель плода, преимущественно в анамнезе у пациенток, не отменяющих антагонист витамина К в I триместре, оказалась достаточно высокой. С другой стороны, по данным Wang J. et al., антагонист витамина K был более эффективен в профилактике тромбозов клапанов в I триместре, чем гепарин. При этом частота самопроизвольных абортов в группах пациенток с двумя различными схемами антикоагулянтной терапии не различалась; это указывало на то, что гепарин не был более безопасным для плода препаратом, чем варфарин.
В то же время в литературе имеются указания на то, что антагонист витамина К более эффективен в профилактике тромбозов клапанов в I триместре, чем гепарин, а частота самопроизвольного аборта между двумя схемами лечения статистически не отличается. Таким образом, гепарин не является более безопасным в I триместре беременности в сравнении с варфарином [12].
В настоящем исследовании продемонстрирована высокая частота оперативного родоразрешения – у 94,1% у пациенток с МПК, что намного превышает имеющиеся европейские данные. По результатам регистра ROPAC, в развивающихся странах кесарево сечение выполнено у 64,7% пациенток с ПКС и в 46,6% случаев в общей популяции рожениц.
В послеродовом периоде основным осложнением у пациенток с протезами клапанов является кровотечение. Частота геморрагических осложнений, по результатам анализа существующих европейских публикаций, варьирует от 2,5 до 33% [9, 10, 14, 19, 20]. Согласно данным проведенного анализа, частота геморрагических осложнений составила 14,7% случаев, в то время как по результатам регистра ROPAC они верифицированы в 23,1%; такой же высокий риск кровотечений регистрировался в Великобритании – у 29% родильниц и, по данным Abildgaard U. et al., в 33% [9, 10, 14].
В отличие от тромботических, геморрагические осложнения как в настоящем исследовании, так и по данным регистра ROPAC не сопровождались прогрессированием сердечной недостаточности, материнской летальностью или потерей плода. Кроме того, согласно результатам регистра ROPAC, кровотечения зарегистрированы в 5,1% случаев у пациенток с БПК и в 4,9% случаев в общей популяции рожениц, тогда как в настоящем исследовании геморрагические осложнения у пациенток с БПК не были установлены.
Проведенный нами анализ подтвердил эффективность подбора дозы НМГ на фоне регулярной оценки анти-Ха активности с достижением целевых уровней в соответствии с существующими рекомендациями по ведению беременных [2, 3]. Также положительное влияние оказало активное внедрение дистанционных телеконсультаций с другими медицинскими центрами. Изменения в тактике ведения позволили исключить одно из грозных осложнений у этой категории пациенток: в течение трехлетнего периода не зарегистрировано ни одного случая тромбоза протеза. Несмотря на это, обращала на себя внимание высокая частота оперативного родоразрешения и геморрагических осложнений в послеродовом периоде. Вероятно, это связано с тем, что до сих пор остается спорным вопрос возможности самостоятельного родоразрешения через 12 ч после введения НМГ из-за несогласованности между Национальными и международными рекомендациями. Так, в Национальных рекомендациях предоставлена возможность родоразрешения через 12 ч после введения НМГ [2], а в Европейских требуется обязательный переход на нефракционированный гепарин (НФГ) за 36 ч до запланированных родов [3]. По нашему наблюдению, высокая частота геморрагических осложнений ассоциирована со временем возобновления терапии антагонистом витамина К в послеродовом периоде. Рекомендованное время возобновления терапии варфарином варьирует по различным данным от 1 до 5 сут. Результаты исследований, демонстрирующих отсутствие материнской летальности у пациенток с геморрагическими осложнениями и их наличие у пациенток с тромбоэмболиями, опосредованно указывают на необходимость более раннего возобновления антикоагулянтной терапии после родов и оперативного родоразрешения. Исходя из результатов нашего анализа, возобновление терапии варфарином должно проводиться не ранее чем на вторые, а оптимально на третьи сутки после оперативного родоразрешения.
Согласно результатам мировых регистров, ретроспективных и проспективных исследований, беременность пациенток с ПКС ассоциируется с высокой частотой материнской летальности, которая варьирует по различным данным от 0,9 до 9% [4, 8–11]. Результаты настоящего исследования, несмотря на высокую частоту тромбоэмболических и геморрагических осложнений, продемонстрировали нулевую материнскую летальность в течение 8 лет наблюдения в группе пациенток как с МПК, так и БПК. В значительной мере этому способствовала локализация перинатального центра в многопрофильном стационаре, обеспечивающем мультидисциплинарный подход с возможностью привлечения при необходимости кардиологов, кардиохирургов, реаниматологов, гематологов к обсуждению и принятию консолидированного решения. Также имели важное значение лабораторная поддержка и возможность определения анти-Ха активности плазмы при введении НМГ. Основной причиной материнской летальности по результатам других исследований были тромбоэмболические осложнения, при этом наиболее неблагоприятный прогноз отмечался у женщин, получающих НМГ, либо НФГ на протяжении всей беременности.
ЗАКЛЮЧЕНИЕ
Отсутствие летальности за 8-летний период наблюдения и родоразрешения у пациенток с ПКС в условиях перинатального центра ФГБУ «НМИЦ им В. А. Алмазова» демонстрирует целесообразность наблюдения и родоразрешения этой категории пациенток в специализированном многопрофильном медицинском учреждении с наличием кардиохиургического отделения, профильного реанимационного отделения, акушерской, педиатрической, гематологической и кардиологической служб с опытом ведения пациенток во время беременности с ПКС на антикоагулянтной терапии. Важной составляющей снижения количества осложнений, улучшения результатов и исходов беременностей служит соблюдение современных рекомендаций по тактике ведения пациенток. Учитывая разночтение по некоторым позициям в разных рекомендациях, целесообразна разработка локальных протоколов ведения беременности у женщин с искусственными клапанами сердца, учитывающих индивидуальные особенности заболевания, возможности телемедицинского сопровождения, лабораторной поддержки, психологической работы с женщинами. В соответствии с переходом на повсеместное использование клинических рекомендаций сотрудники специализированных перинатальных центров должны активно включаться в работу по их гармонизации в составе профессиональных сообществ.



