ВВЕДЕНИЕ
В структуре смертности в России до 46,8% занимают болезни системы кровообращения (БСК). Смертность от БСК по стране в целом равняется 583,1 на 100 тыс. населения, в Архангельской области – 749,9 на 100 тыс. жителей; при этом целевое значение этого показателя в соответствии с национальным проектом «Здравоохранение» составляет 565 на 100 тыс. [1, 2]. Наиболее распространенными заболеваниями системы кровообращения, представляющими непосредственную угрозу для жизни пациента, являются тромбозы и тромбоэмболические осложнения. Восьмилетняя выживаемость пациентов, которые перенесли тромбоз глубоких вен (ТГВ), составляет 62,5%, а после тромбоэмболии легочной артерии (ТЭЛА) – 34,5%, что значимо ниже средней прогнозируемой продолжительности жизни в общей популяции [3].
В настоящее время для профилактики и лечения тромбозов, тромбоэмболических осложнений в реальной клинической практике широко используются оральные антикоагулянты – антагонисты витамина К (АВК), в частности варфарин, и прямые пероральные антикоагулянты (ПОАК). Важно отметить, что продленная (пожизненная) антитромботическая терапия (АТТ) значительно повышает риск возникновения геморрагических осложнений. Развитие серьезного кровотечения при приеме оральных антикоагулянтов может вызвать не только передозировка препарата, но и сопутствующие заболевания, отсутствие приверженности к фармакотерапии, а также фармакогенетические особенности пациента [4].
Цель исследования – анализ возможных причин развития внутричерепных геморрагических осложнений на фоне продленной оральной антикоагулянтной терапии в реальной клинической практике.
МАТЕРИАЛ И МЕТОДЫ
В проспективное клиническое исследование включен анализ внутричерепных геморрагических осложнений у пациентов с продленной АТТ, госпитализированных в ГБУЗ АО «Первая городская клиническая больница им. Е.Е. Волосевич» г. Архангельска в период 2014–2021 гг. Исследование выполнено на базе кафедры клинической фармакологии и фармакотерапии ФГБОУ ВО «Северный государственный медицинский университет» (СГМУ) Минздрава России (г. Архангельск). Сбор анамнеза и биологических данных проводился по правилам международного стандарта этических норм и качества научных исследований (CGP).
Изучалось наличие вероятных факторов, которые могут приводить к развитию геморрагический осложнений, исходы осложнений и их частота, данные о лекарственном препарате, показания для назначения АТТ, сопутствующие заболевания и их фармакотерапия, уровень международного нормализованного отношения (МНО) для пациентов, получающих АВК, особенности фармакогенетического профиля пациента, а также уровень артериального давления (АД) при госпитализации в стационар.
Материалом для лабораторного исследования стали образцы венозной крови, полученные путем венепункции локтевой вены при поступлении пациента в стационар. Кровь набиралась самотеком, с использованием пробирок VACUETTE, содержащих антикоагулянт – 3,2% раствор цитрата натрия для гемостазиологических исследований, в вакутейнер с этилендиаминтетрауксусной кислотой (ЭДТА) для фармакогенетического исследования методом полимеразной цепной реакции (ПЦР). Для определения полиморфизма генов ABCG2, MDR1(C/T), MDR1(G/T), CYP3A5, CYP3A4 использовался метод ПЦР в режиме реального времени (ПЦР-РВ) на амплификаторе Real-Time CFX96 c применением набора реагентов «SNP-Cкрин».
Протокол исследования был одобрен локальным этическим комитетом СГМУ.
Статистическая обработка данных, полученных в ходе исследования, осуществлялась при помощи описательной и аналитической статистики, с использованием программы IBM SPSS Statistics 23.0.
РЕЗУЛЬТАТЫ
В анализ были включены 83 пациента (38 женщин и 45 мужчин) в возрасте Me=67,4 [46; 83] лет, госпитализированных в отделение нейрореанимации регионального сосудистого центра с диагнозом «внутримозговое/субарахноидальное кровоизлияние», подтвержденным клинически и при компьютерной томографии (КТ) головного мозга. Все пациенты перед поступлением в стационар получали продленную АТТ оральными антикоагулянтами. У всех участников дополнительно были проанализированы амбулаторные карты из поликлиник по месту их наблюдения.
Показаниями для назначения продленной АТТ в исследуемой группе были фибрилляция предсердий (ФП), механический протез клапанов сердца, ТГВ или посттромбофлебитический синдром, ТЭЛА, тромбоз артерий (рис. 1).

Варфарин получали 60 пациентов (72%), ПОАК – 23 (28%), в том числе 12 человек – апиксабан, 10 – ривароксабан, 1 – дабигатрана этексилат.
Среди всех пациентов 60% были заняты трудовой деятельностью, 40% не работали. На все случаи зарегистрированных геморрагических осложнений на фоне АТТ были заполнены формы извещения о побочном действии препаратов и направлены в Архангельский областной центр мониторинга безопасности лекарственных средств. Летальный исход на фоне развившегося внутримозгового кровоизлияния (ВМК) был отмечен в 44% случаев.
Анализ коморбидности пациентов выявил следующие сопутствующие заболевания: анемию, патологию щитовидной железы, нарушения функции почек или печени. Важно подчеркнуть, что нарушение функции почек было выявлено у 74% от общего числа пациентов, что еще раз свидетельствует о важности контроля состояния почечной функции на фоне АТТ. Нарушение функции печени было зарегистрировано в 28%, анемия – в 18%, патология щитовидной железы – в 6% случаев. Черепно-мозговая травма перед поступлением в стационар была установлена у 6% пациентов.
Анализ показал, что 93,5% пациентов при поступлении имели артериальную гипертензию (АГ) без достижения целевого значения АД (в 60,9% случаев при поступлении была зафиксирована высокая АГ – 3-й степени), требующую фармакотерапии антигипертензивными препаратами.
Сопутствующая терапия была представлена лекарственными препаратами, взаимодействующими на уровне цитохромов 450 с используемыми антитромботическими средствами. В структуре сопутствующей терапии преобладали статины, нестероидные противовоспалительные препараты (НПВП), ингибиторы протонной помпы (рис. 2).
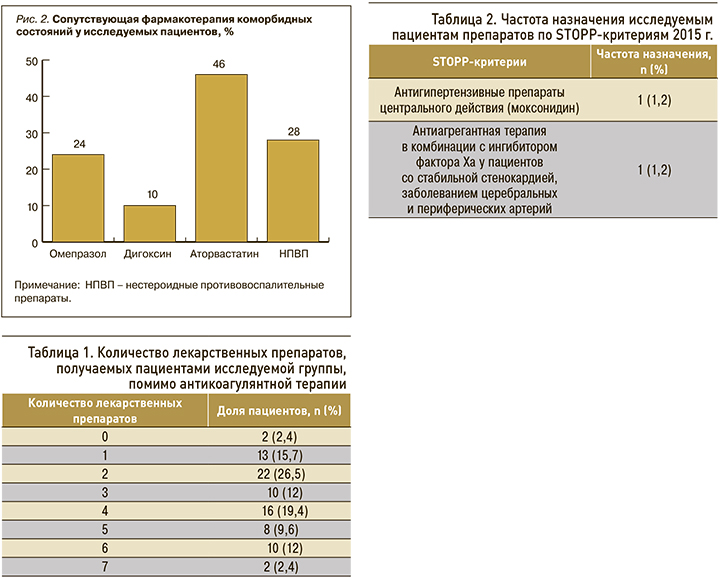
В ходе исследования было проанализировано количество наименований лекарственных препаратов, назначенных пациентам помимо АТТ, и наличие STOPP-критериев. Кроме антикоагулянтов, пациенты получали до 7 лекарственных препаратов, что могло служить дополнительным фактором развития побочных реакций. Количество лекарственных средств, получаемых пациентами исследуемой группы (не считая оральных антикоагулянтов), представлено в таблице 1.
В анализируемой группе до поступления в стационар антигипертензивную терапию получали 49 пациентов, противоаритмические средства – 17, ацетилсалициловую кислоту – 16, клопидогрел – 12, статины – 37, препараты фолиевой кислоты – 23, венотонизирующие средства – 27, сахароснижающие препараты – 15, глюкокортикостероиды – 12, синтетические аналоги тироксина – 16. По данным историй болезни, у пациентов старше 65 лет был проведен анализ STOPP-критериев редакции 2015 г. Результаты этого анализа приведены в таблице 2.
Таким образом, назначение потенциально не рекомендованных лекарственных препаратов согласно STOPP-критериям редакции 2015 г. было выявлено у 2 пациентов из обследованной группы.
Лабораторный мониторинг медикаментозной гипокоагуляции на фоне терапии варфарином показал, что на момент госпитализации в стационар уровень МНО превышал 3,0 единицы в 91,67% случаев, что свидетельствовало о чрезмерном уровне наведенной медикаментозной гипокоагуляции. У 3 пациентов, получающих варфарин, был отмечен терапевтический диапазон показателя МНО (2–3 единицы) на фоне некорригируемой АГ, что стало непосредственной причиной ВМК. Следует добавить, что все пациенты получали оригинальный препарат варфарина.
Результаты генотипирования пациентов, включенных в исследование, суммированы в таблице 3.
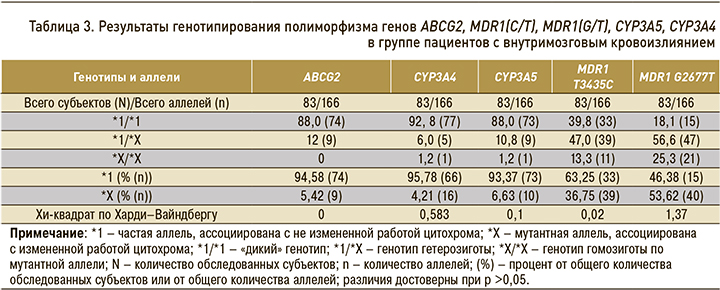
Результаты фармакогенетического анализа показали следующее: в гене ABCG2 аллельный вариант АА (мутантный тип) в данной группе пациентов отсутствовал; в генах CYP3A4 и CYP3A5 генотип АА (мутантный тип) был обнаружен у 1 пациента (1,2%); в гене MDR1 T34354C (rs1045642) генотип СС (мутантный тип) был выявлен у 11 пациентов (13,4%); наибольшая частота мутантного аллеля была отмечена в гене MDR1 G2677T (rs2032582) – у 21 (25,3%) пациента (рис. 3).
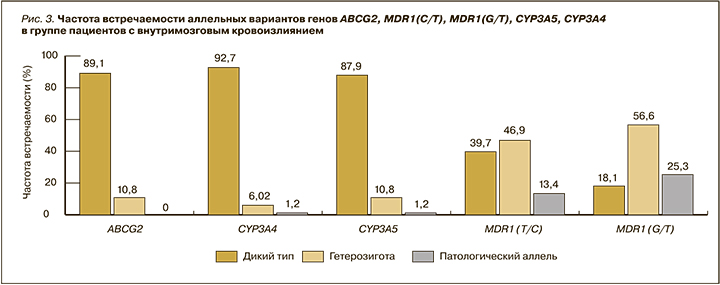
В группе пациентов с ВМК полиморфизм генов MDR1(C/T), MDR1(G/T), возможно, оказывал влияние на развитие кровотечения.
Важно отметить, что большая часть пациентов (80%) не посещала школу приема антикоагулянтов (варфаринотерапии или ПОАК) для формирования приверженности к терапии, несмотря на наличие антикоагулянтного кабинета в медицинской организации по месту их жительства.
ОБСУЖДЕНИЕ
Обладая высокой эффективностью в профилактике тромбоэмболических событий, продленная АТТ в то же время может привести к серьезному осложнению в виде массивного или жизнеугрожающего кровотечения. Согласно результатам проспективных исследований, частота больших кровотечений в реальной клинической практике достигает 0,3–4,2% в год [5, 6].
Эффективность и безопасность терапии АВК и ПОАК, предупреждение развития нежелательных побочных реакций по большей части зависят не только от самого лекарственного средства, но и от определяемых индивидуальными особенностями организма фармакодинамики и фармакокинетики препарата. Поскольку около 30% выведения препарата из организма для апиксабана и 50% для ривароксабана осуществляется с почечной экскрецией, важным при назначении ПОАК и контроле терапии представляется определение скорости клубочковой фильтрации (СКФ) [7, 8].
Частота развития нежелательных побочных реакций в России в амбулаторных условиях варьирует от 10 до 20%, а в условиях стационара может достигать 17–30%. В группе пациентов пожилого возраста их частота может повышаться до 41,9% в амбулаторных условиях и до 50% на госпитальном этапе [5, 21]. Одной из причин развития нежелательных побочных реакций, помимо индивидуальных особенностей организма, является единовременное назначение широкого спектра лекарственных средств – полипрагмазия. По данным фармакоэпидемиологических исследований, у одного пожилого пациента наблюдается в среднем от 2 до 4 заболеваний, вследствие чего таким пациентам назначается большее количество лекарственных препаратов, чем пациентам более молодого возраста [9–11].
По результатам нашего анализа, при проведении антикоагулянтной терапии следует учитывать ряд факторов. Так, в условии сниженной клубочковой фильтрации, сахарного диабета, АГ и хронической сердечной недостаточности АВК может длительно задерживаться в организме, вызывая кумулятивный эффект, что усиливает терапевтическое воздействие антикоагулянта [12]. Необходимо учитывать, что ПОАК также выводятся почками, поэтому для обеспечения максимальной безопасности лечения необходимо регулярно следить за функцией этих органов.
Немаловажно, что в проведенном нами исследовании нарушение функции почек было выявлено у 74% от общего числа пациентов. Этот факт имеет особое значение при наличии у пациента фармакогенетических особенностей. Так, известно, что ген ABCB1 (в большинстве источников известный как MDR1 – multidrug resistance) является геном множественной лекарственной устойчивости [13]. К наиболее изученным относится однонуклеотидный полиморфизм C3435T, результат которого выражается в снижении синтеза мРНК и белка. Проявлением такой замены становится уменьшение экспрессии P-гликопротеина на поверхности клетки. Вследствие этого наблюдается сниженный клиренс лекарственных веществ, служащих субстратами для Р-гликопротеина, что особо значимо при хронической болезни почек [14].
Немалый интерес представляет проведенное в России исследование, в котором приняли участие пациенты ортопедических отделений, госпитализированные по поводу эндопротезирования коленных и тазобедренных суставов. С целью профилактики тромбогеморрагических осложнений эти пациенты получали ривароксабан. В результате выяснилось значимое влияние полиморфизма ABCB1 3435C>T (rs1045642) на характер изменения протромбинового времени у исследуемых пациентов, что при нарушении почечной функции может привести к кровотечению [15].
На эффективность и безопасность терапии варфарином и некоторыми ПОАК, имеющими печеночный путь элиминации, также влияет функция печени, что объясняется механизмом действия препаратов и их метаболизмом. Дополнительно установлено, что при повреждении паренхимы печени происходит вытеснение варфарина из комплекса с белками крови, что усиливает его антикоагулянтный эффект. В проведенном нами исследовании нарушение функции печени было выявлено у 28% пациентов.
Частота развития кровотечений во многом зависит от достижения и времени нахождения МНО в границах терапевтического диапазона: относительный риск внутричерепных кровотечений при МНО >3,0 достоверно возрастет в 4,6 раза, а при более высоких его значениях – в 8,8 раз (у пожилых – в 19,3 раза) [16]. В нашем исследовании на момент госпитализации значение МНО >3,0 было зарегистрировано у 91,67% пациентов.
Стоит отметить, что на момент госпитализации 93,5% пациентов имели АГ без достижения целевого значения АД (у 60,9% была диагностирована АГ 3-й степени, что, как правило, является кризом), требующую коррекции фармакотерапии. Именно этот факт может быть ключевым в развитии ВМК.
ЗАКЛЮЧЕНИЕ
Исходя из литературных данных и результатов проведенного анализа, причинами, способствующими развитию внутричерепного геморрагического осложнения на фоне продленной АТТ, можно считать следующие факторы: превышение показателя МНО более 3,0 при терапии АВК; сопутствующую патологию печени, почек, щитовидной железы, анемию, АГ без достижения целевых показателей АД, особенно ее кризовое течение; наличие полиморфизма в гене MDR1 – multidrug resistance. Эти факторы имели место в анамнезе изученной группы пациентов с развившимся серьезным кровотечением при продленной АТТ.
«Лекарства не будут работать, если их не принимать» – таков неоспоримый постулат из отчета Всемирной организации здравоохранения, в котором приверженность к терапии определяется как главный залог успешного лечения пациента [17]. В развитых странах только 50% пациентов, страдающих хроническими заболеваниями, придерживаются рекомендаций по лечению. Если же говорить о развивающихся странах, то в них из-за ограниченного доступа населения к медицинской помощи, отсутствия надлежащей диагностики и недостатка снабжения лекарственными средствами плохая приверженность пациентов к терапии и вовсе угрожает сделать бесполезными государственные усилия по борьбе хроническими заболеваниями [17, 18]. Недостаточная приверженность к медикаментозной терапии может приводить к таким неблагоприятным последствиям, как кровотечение, развитие фатальных осложнений, ухудшение состояния пациента, повышение смертности и, следовательно, вызывать увеличение затрат здравоохранения.
К причинам снижения приверженности пациента к лечению в реальной клинической практики можно отнести забывчивость или сознательное решение пропустить дозу, недостаток верной информации и отсутствие понимания необходимости назначенной терапии, эмоциональные факторы, сложность режима дозирования препаратов. Большую часть этих причин можно скорректировать с помощью таких мер, как объяснение важности для пациента приема лекарственных препаратов, использование максимально возможных простых режимов дозирования, адаптация режима терапии к образу жизни пациента, наблюдение за больным, регулярные его визиты к врачу [19].
Для оценки уровня приверженности пациента к лечению может применяться метод пропорции покрытых дней (Proportion of Days Covered, PDC), разработанный Pharmacy Quality Alliance (США). Показатель PDC представляет собой отношение дней, в которые пациент фактически принимал препарат, к количеству дней, на которые был выписан препарат по рецепту (результат выражается в процентах). Приверженными к терапии признаются пациенты с PDC >80%. Определение этого показателя одобрено в качестве нового метода оценки эффективности использования ПОАК. Считается, что он предпочтителен для оценки пролонгированной терапии, однако может быть не самым подходящим методом для оценки приверженности к терапии в случае ее короткой продолжительности [19–21]. Необходимо уделять особое внимание приверженности пациентов к лечению, так как повышение ее уровня служит одним из действенных способов снижения затрат ресурсов здравоохранения.
С целью формирования приверженности к пролонгированной антикоагулянтной терапии ПОАК для уменьшения риска развития осложнений целесообразно обучение пациентов в школах антикоагулянтной терапии, проводимых в антикоагулянтных кабинетах (клиниках).



