ВВЕДЕНИЕ
Начиная с марта 2020 г., в странах Европы и США стали появляться сообщения о случаях заболевания, которое развивалось у детей после перенесенной (в том числе бессимптомно) новой коронавирусной инфекции COVID-19 и сопровождалось развитием выраженного гипервоспалительного синдрома. Клинически это заболевание соответствовало ряду критериев болезни Кавасаки, у некоторых детей наблюдалась клиника синдрома токсического шока, синдрома активации макрофагов или миокардита с кардиогенным шоком [1–5].
Всемирной организацией здравоохранения (ВОЗ) на основании анализа этих случаев уже в мае 2020 г. было дано определение мультисистемного воспалительного синдрома у детей, ассоциированного с COVID-19 (MIS-С) [6].
После того как MIS-C был идентифицирован у детей, многие врачи стали отмечать характерные для него проявления и у взрослых. Таким образом, был сделан вывод, что состояние, ранее описанное только в педиатрической практике, может развиваться после перенесенной новой коронавирусной инфекции COVID-19 в разных возрастных группах [7, 8].
Мультисистемный воспалительный синдром взрослых, ассоциированный с Covid-19 (MIS-A), – редкое жизнеугрожающее иммунопатологическое осложнение новой коронавирусной инфекцией COVID-19, развивающееся у лиц старше 21 года в течение 12 нед от начала заболевания и характеризующееся развитием тяжелого поражения ≥1 органа или системы, за исключением органов дыхания, и наличием лабораторных признаков системного воспаления [9].
Диагностика MIS-A затруднена по причине схожести его клинических проявлений с системной гипериммунной реакцией, которая может иметь место при продолжающемся симптоматическом COVID-19 или постковидном синдроме, а также из-за отсутствия точных данных о временной связи между MIS-A и острым периодом COVID- 19. С практической точки зрения представляется актуальным обобщение имеющихся к настоящему времени данных о патогенезе, клинических проявлениях, особенностях течения, критериях диагноза и лечении MIS-A.
ЭПИДЕМИОЛОГИЯ И ОСНОВНЫЕ ДЕМОГРАФИЧЕСКИЕ ХАРАКТЕРИСТИКИ ПАЦИЕНТОВ С MIS-A
Точных данных о распространенности MIS-A в настоящее время нет, однако предположительно развивается он достаточно редко. В систематическом обзоре Patel P. et al. (2021) приводятся результаты анализа 221 случая MIS-A, согласно которому большая часть пациентов была лицами мужского пола в возрасте от 19 до 34 лет [10]. По данным Vogel T.P. et al. (2021), MIS-A чаще развивается у лиц в возрасте до 50 лет с сопутствующими заболеваниями [11]. Средняя продолжительность госпитализации после этого заболевания составила 8 дней. В большинстве случаев отмечалось тяжелое течение MIS-A: у 51% пациентов имела место клиника шока/гипотензии с применением вазопрессоров, 57% были госпитализированы в отделение реанимации и интенсивной терапии, в 47% случаев потребовалась респираторная поддержка (в половине из этих случаев искусственная вентиляция легких), летальность составила 7% [10].
ПАТОГЕНЕЗ
Патофизиология MIS-A изучена недостаточно. Известно, что острый период COVID-19 у взрослых может протекать тяжело, с развитием острого респираторного дистресс-синдрома (ОРДС), при этом триггером быстрого прогрессирования и полиорганной недостаточности выступает системный гипервоспалительный синдром. Развитие MIS-A характеризуется гипериммунной воспалительной реакцией с высвобождением цитокинов, обладающей сходством с синдромом активации макрофагов. Определенную роль, возможно, играет длительная экстрапульмональная персистенция вируса, который, как известно, обнаруживается во многих органах и тканях, включая сердце, печень, головной мозг, почки, желудочно-кишечный тракт (ЖКТ). Важное значение может иметь вызванное вирусом SARS-CoV-2 повреждение эндотелия, тромботическая микроангиопатия, дисрегуляция иммунного ответа и ренин-ангиотензин-альдостероновой системы (РААС) [7].
Эндотелиальная дисфункция – один из основных патогенетических механизмов тяжелого течения острого периода COVID-19 [12]. Повреждение эндотелия, вызванное прямым действием вируса или развивающееся в рамках системного воспаления, приводит к гиперкоагуляции, коагулопатии и тромбоэмболическим осложнениям как в остром периоде коронавирусной инфекции, так и в постковидном периоде, в том числе при развитии MIS-A [13–16]. Особенности иммунного ответа у пациентов с MIS-A требуют дальнейших исследований, однако существующие данные уже позволяют говорить о том, что описанный механизм является одним из основных.
Есть предположение, что развитие MIS-A – следствие отсроченной реакции системы иммунитета [17]. Так, в развитии мультисистемного воспалительного синдрома у детей, ассоциированного с COVID-19 (MIS-C), уже доказана роль снижения количества нейтрализующих антител и их ограниченной функциональности, что приводит к персистирующему инфекционному воспалению в органах и тканях, и аутоантител, выступающих в роли промоторов воспаления [18]. То же самое может происходить и у взрослых, при этом у них дисбаланс между противовирусным и провоспалительным ответом организма, вызывающий гипервоспаление, усугубляется возрастом и сопутствующей патологией [19, 20].
Очевидно, что большое значение в развитии агрессивной воспалительной реакции и иммуноопосредованном повреждении органов и тканей может иметь экстрафолликулярная активация В-лимфоцитов [21, 22]. Активация моноцитов и натуральных киллеров, продуцирующих большое количество провоспалительных цитокинов, влечет за собой как «цитокиновый шторм», так и к дисрегуляцию РААС [23–26].
Известно, что тяжесть течения и летальность при COVID-19 коррелируют со степенью лимфопении, при которой снижается число как CD4+, так и CD8+ субпопуляций Т-лимфоцитов. Причина лимфопении до конца неизвестна; возможно, определенную роль здесь играет прямое повреждающее действие вируса на Т-лимфоциты, как при MERS-CoV, или влияние системного воспаления на секвестрацию Т-лимфоцитов [27–30]. И если индукция адекватного Т-клеточного иммунитета является обязательной для формирования эффективного противовирусного иммунитета, то дисрегуляция Т-клеточного ответа может вносить определенный вклад в развитие гипериммунного воспаления [31]. Патоморфологические исследования подтверждают вовлеченность в патологический процесс органов иммунной системы, в которых выявлен широкий диапазон изменений от выраженного опустошения, напоминающего изменения при ВИЧ-инфекции на стадии СПИД, до разной степени гиперплазии, преимущественно Т-зависимых, так и реже B-зависимых зон лимфоидной ткани. Так же, как и в легких, в краевых синусах лимфатических узлов был обнаружен феномен аутоцитофагии, от гемоцитофагии до фагоцитоза макрофагами фрагментов и целых лимфоцитов [32].
По аналогии с MIS-C важную роль в развитии MIS-A может играть нарушение интерферонового статуса с гиперпродукцией интерферонов гамма (IFN-γ) [33].
Для понимания иммунопатогенеза MIS-A необходимы дальнейшие исследования, направленные в том числе на выявление специфических иммунологических маркеров этого патологического процесса.
КЛИНИЧЕСКАЯ КАРТИНА
По данным Patel P. et al. (2021), к основным клиническим проявлениям MIS-A относятся лихорадка (96%), гипотензия (60%), нарушения работы сердца (54%), одышка (52%) и диарея (52%). Наиболее часто поражаются система крови (92%), сердечно-сосудистая система (87%), ЖКТ (83%) и органы дыхания (74%). У 61 из 205 пациентов был диагностирован миокардит, что составило 30%, у 44 из 175 (25%) выявлен выпот в перикарде. В 5% случаев наблюдались артериальные или венозные тромбозы [10]. У одного пациента, согласно данным литературы, развились тяжелый неврит правого срединного и лицевого нервов и двустороннее поражение локтевого, большеберцового, малоберцового и грудного нервов с миокардитом и кардиогенным шоком [34].
В качестве наиболее важных клинических критериев диагноза MIS-A определены симптомы поражения кожи и слизистых оболочек (сыпь, эритема, растрескивание губ и слизистой полости рта, двусторонний конъюнктивит, отеки и эритема кистей и стоп), ЖКТ (боли в животе, рвота, диарея), а также шок или гипотензия, поскольку именно эти проявления наблюдались у большинства пациентов [3, 7, 35–39]. В перечень клинических критериев MIS-A включены и неврологические симптомы (нарушение сознания, головная боль, слабость, парестезии, сонливость): они, хоть и не являются частыми, однако достаточно специфичны. В то же время в этот перечень не вошли симптомы поражения органов дыхания, в связи с тем что не они обычно определяют тяжесть состояния больного [35, 37, 38]. Более того, наличие тяжелых респираторных симптомов, согласно действующему определению, исключает диагноз MIS-A [11].
ЛАБОРАТОРНЫЕ ДАННЫЕ
В большинстве описанных к настоящему времени случаев при MIS-A отмечалось возрастание уровней Д-димера (91%), лимфопения (86%), повышение маркеров коагулопатии и/или системного воспаления (90%), таких как интерлейкин 6 (ИЛ-6), ферритин, фибриноген, С-реактивный белок (СРБ), натрийуретический пептид. У каждого пациента с MIS-A наблюдалось увеличение уровня хотя бы одного из следующих показателей: ИЛ-6 (98%), ферритина (91%), фибриногена (91%), СРБ (90%), натрийуретического пептида В-типа (BNP) (74%) или натрийуретического гормона (В-типа) N-концевого пропептида (NT-proBNP) (90%). Средние значения маркеров воспаления составили для ИЛ-6 – 86 пг/мл (референсные значения ≤1,8 пг/мл), для ферритина – 1029 нг/ мл (референсные значения 12–300 нг/мл для мужчин и 12–150 нг/мл для женщин), для СРБ – 24 мг/дл (референсные значения 0–10 мг/дл), для фибриногена – 624 мг/дл (референсные значения 200–400 мг/дл), для BNP – 271 пг/мл (референсные значения <100 пг/мл) и для NT-proBNP – 2219 нг/л (референсные значения <125 нг/л) [10].
К настоящему времени очевидно, что наиболее типичными лабораторными признаками MIS-A являются нейтрофилия, лимфопения и тромбоцитопения. Эти показатели, наряду с тропонином и BNP/NT-proBNP, служат критериями вовлеченности в патологический процесс системы крови и сердечно-сосудистой системы и используются для мониторинга активности заболевания [3, 7, 35–39]. Активность воспаления оценивается по повышению уровней СРБ, скорости оседания эритроцитов (СОЭ), ферритина или прокальцитонина не потому, что другие маркеры воспаления (такие как Д-димер, ИЛ-6 или лактатдегидрогеназа) не возрастают при MIS-A, а потому, что изолированное повышение различных маркеров без увеличения СРБ, СОЭ, ферритина или прокальцитонина, согласно действующим рекомендациям, не имеет диагностического значения [7, 35].
ДИФФЕРЕНЦИАЛЬНАЯ ДИАГНОСТИКА
Учитывая неспецифический характер клинических проявлений и лабораторных данных, MIS-A необходимо дифференцировать с достаточно широким кругом инфекционно-воспалительных заболеваний. При этом своевременная дифференциальная диагностика представляется крайне важной, так как от ее результатов зависит ведение пациентов, которое может существенно различаться.
У детей MIS-C дифференцируют прежде всего с болезнью Кавасаки. Известно, что это заболевание крайне редко встречается у взрослых; в то же время определенные ее признаки наблюдаются при MIS- A, поэтому необходимо сделать акцент на особенностях анамнеза и клиники заболевания пациента, не свойственных болезни Кавасаки [7]. К примеру, симптомы поражения ЖКТ, типичные для MIS-A, такие как абдоминальные боли, рвота, диарея, для болезни Кавасаки не характерны [40]. В отличие от этой болезни при MIS-A часто наблюдается ожирение [5, 35, 41]. Также очевидно, что для MIS-А более типичны высокие уровни маркеров воспаления, тропонина и BNP или NT-proBNP, более выражены анемия, лимфопения и тромбоцитопения [3, 5, 7, 36–38, 41, 42]. При MIS-A чаще развиваются шок или гипотензия, требующие применения вазопрессоров, изменения на электрокардиограмме (ЭКГ) [36, 42, 43]. Частота развития шока при болезни Кавасаки составляет не более 5%, тогда как при MIS-A этот показатель равен 80% [36–38, 42, 44].
Кожные симптомы MIS-A (полиморфные высыпания) необходимо дифференцировать с синдромом токсического шока стафилококковой или стрептококковой этиологии [3, 35–38, 45, 46]. К общим проявлениям этих патологических состояний относятся лихорадка, шок, кожные сыпи, а вот конъюнктивит чаще наблюдается при стафилококковом шоке [47]. Абдоминальные симптомы являются типичными для MIS-A, при этом профузная диарея с гипотензией чаще сопутствует стафилококковому, чем стрептококковому токсическому шоку [35–38, 45, 48]. Для шока не характерны такие часто наблюдаемые при MIS-A проявления, как головная боль и респираторные симптомы [35, 38, 45]. При стафилококковом синдроме ошпаренной кожи и других стафилококковых эксфолиативных дерматитах определяется положительный симптом Никольского. При скарлатине наблюдается типичная эритематозная сыпь, наощупь напоминающая наждачную бумагу. Для стрептококковых инфекций характерен «малиновый язык», при MIS-A губы обычно нормальной окраски, а в ротоглотке обнаруживаются тонзиллярный экссудат и нёбные петехии.
Многие бактериальные инфекции могут иметь признаки, сходные с MIS-A, однако в отличие от MIS-A большая часть из них обычно протекает с вовлечением только одного органа или системы. Некоторые тяжелые системные инфекции, такие как лептоспироз или риккетсиозы, могут сопровождаться лихорадкой, шоком, высыпаниями, что требует дифференциальной диагностики с учетом географических особенностей, используемой населением воды, контактов с животными, насекомыми и клещами [49].
При дифференциальной диагностике с вирусными инфекциями необходимо помнить, что последние, как и MIS-A, в большинстве случаев могут протекать с лихорадкой. Примеры – энтеровирусные, аденовирусные, парвовирусные, герпесвирусные инфекции. Конъюнктивит может развиваться при кори, краснухе, аденовирусной и хантавирусной инфекциях [50]. Симптомы со стороны ЖКТ, типичные для MIS-A, также характерны для аденовирусов, энтеровирусов, ротавирусов и вируса Норуолк; при этом абдоминальные боли при MIS-A могут быть значительно интенсивнее, как при остром аппендиците [36]. Вирусные инфекции, как и MIS-A, могут протекать с мультисистемным поражением. Так, вирус Эпштейна–Барр (ВЭБ) способен вызывать поражение центральной нервной системы, печени, легких и сердца. ВЭБ и другие вирусы могут индуцировать гипериммунное воспаление, сходное с таковым при MIS-A [7, 37–39, 43, 52]. Также многие вирусы могут оказывать прямое токсическое действие на кардиомиоциты и индуцировать миокардиты, приводящие к сердечной недостаточности [52], в то время как при MIS-A дисфункция миокарда, скорее всего, носит транзиторный характер и, как правило, разрешается без последствий [36]. Некоторые кожные и системные симптомы MIS-A также похожи на проявления синдрома Стивенса–Джонсона, токсического эпидермального некролиза и реакций лекарственной гиперчувствительности с эозинофилией и системными проявлениями. В данном случае нужно помнить, что при синдроме Стивенса–Джонсона, токсическом эпидермальном некролизе поражение кожи бывает гораздо более значительным, часто определяется положительный симптом Никольского. Поскольку во всех этих случаях возможно мультиорганное поражение и шок, необходимо уделить особое внимание анамнезу и при необходимости провести биопсию кожи [53, 54].
ДИАГНОСТИЧЕСКИЕ КРИТЕРИИ И ОПРЕДЕЛЕНИЕ СЛУЧАЯ MIS-A
Vogel T.P. et al. (2021) предложены диагностические критерии рассматриваемого заболевания. В соответствии с ними определенный случай MIS-A можно диагностировать при наличии следующих критериев:
- возраст от 21 года и старше;
- персистирующая лихорадка в течение ≥3 дней;
- ≥2 клинических признаков: поражение кожи и слизистых; поражение ЖКТ; шок, гипотензия; неврологические симптомы; миокардит и др.;
- лабораторные маркеры воспаления и коагулопатии (СРБ, Д-димер, фибриноген, ИЛ-6 и др.);
- ≥2 признаков активности заболевания: повышение уровней NaproBNP и/или тропонина; нейтрофилия, лимфопения и/или тромбоцитопения; изменения на ЭКГ и при эхокардиографии (ЭхоКГ);
- подтвержденная связь с новой коронавирусной инфекцией COVID-19.
Подтверждением связи заболевания с COVID- 19 могут служить лабораторное подтверждение инфекции, вызванной SARS-CoV-2; личный анамнез подтвержденной новой коронавирусной инфекции в течение предшествующих 12 нед; контакт с пациентом с подтвержденной инфекцией COVID-19 в течение предшествующих 12 нед; предшествующая вакцинация против SARS-CoV-2 (табл.) [11].
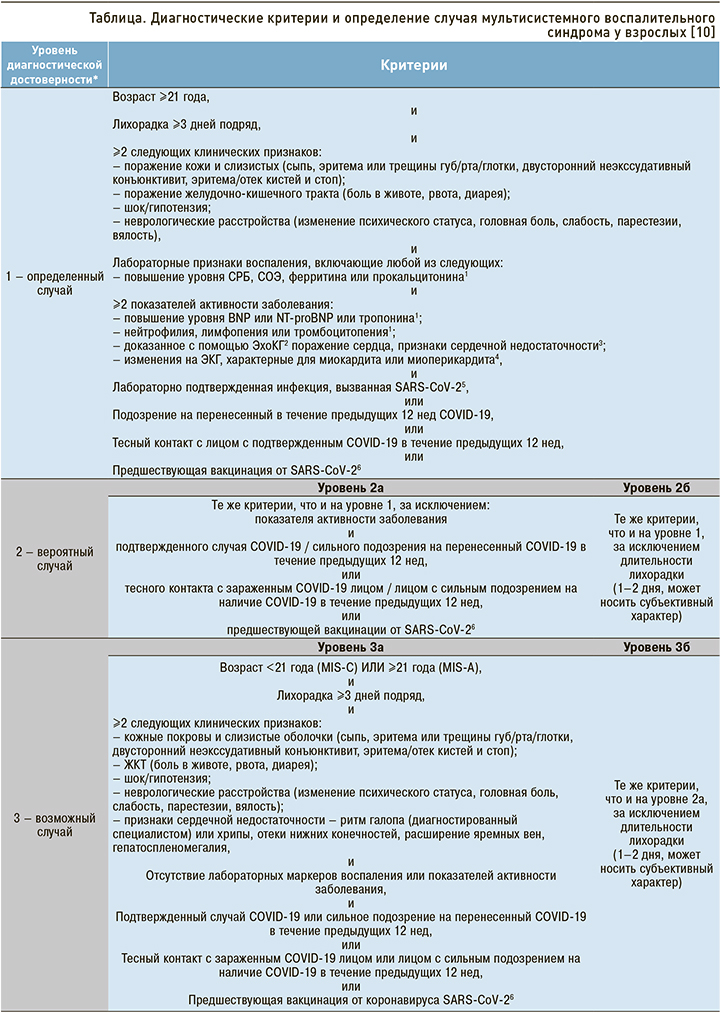

У пациентов, которые не соответствуют критериям определенного случая MIS-A, следует исключать другие заболевания, продолжая при этом наблюдение и лечение, особенно в случае подтвержденной связи (в том числе эпидемиологической) с SARS-CoV-2. Также необходимо дифференцировать этот синдром от волнообразного течения острого периода COVID-19. В этом могут помочь повторные исследования на РНК SARS-CoV-2 методом полимеразной цепной реакции (ПЦР) и серологические тесты на наличие антител. Однако нужно иметь в виду, что у ряда коморбидных больных образование антител может быть более поздним и в течение длительного времени может наблюдаться положительный тест на РНК SARS-CoV-2.
ТЕРАПИЯ
В настоящее время нет каких-либо четких рекомендаций по лечению MIS-A, основанных на принципах доказательной медицины. Базовые терапевтические мероприятия включают применение системных глюкокортикостероидов (ГКС) и/ или препаратов иммуноглобулинов для внутривенного введения в качестве терапии первой линии, генно-инженерных биологических препаратов (ингибиторов ИЛ-1, ИЛ-6) – в качестве второй линии, а также антикоагулянтов и антиагрегантов. По имеющимся в литературе данным, для лечения пациентов с установленным диагнозом MIS-A к настоящему времени в 57% случаев применялись антикоагулянты (гепарин, низкомолекулярные гепарины), в 74% – системные ГКС (дексаметазон и др.), в 55% – иммуноглобулины для внутривенного введения, в 21% – иммуномодуляторы (тоцилизумаб и др.) [10].
Далее мы приводим наше собственное клиническое наблюдение случая мультисистемного воспалительного синдрома у взрослых (MIS-A).
ОПИСАНИЕ КЛИНИЧЕСКОГО СЛУЧАЯ
Женщина, 50 лет, была госпитализирована 25.02.2021 в инфекционное отделение с жалобами на повышение температуры тела до 39,2 °С, выраженную общую слабость, головную боль, головокружение, тошноту, рвоту, диарею, высыпания на нижних конечностях, боли в пояснице и грудной клетке.
Анамнез болезни: за три дня до госпитализации у пациентки появились лихорадка, рвота до 3–4 раз/ сут, диарея (частота стула 3–6 раз/сут), сыпь. За медицинской помощью не обращалась, принимала парацетамол, ибупрофен, хлоропирамин, энтеросгель. Госпитализирована в экстренном порядке в связи с ухудшением состояния.
С 12.01.2021 по 26.01.2021 находилась на стационарном лечении с диагнозом «новая коронавирусная инфекция COVID-19, лабораторно подтвержденная U07.1. Двусторонняя полисегментарная пневмония средней степени тяжести». Проведенное лечение: дексаметазон, эноксапарин, бисопролол, спиронолактон, амлодипин, цефтриаксон, омепразол. Была выписана в удовлетворительном состоянии с рекомендациями по приему апиксабана по 2,5 мг внутрь 2 раза/сут в течение месяца.
Анамнез жизни: росла и развивалась соответственно возрасту. Травм, операций не было. Гинекологический анамнез без особенностей.
Сопутствующие заболевания: ишемическая болезнь сердца, атеросклеротический кардиосклероз, гипертоническая болезнь II ст., риск 3 (высокий), НIIА, ожирение I степени. Пациентка ежедневно принимает бисопролол (5 мг/сут), спиронолактон (50 мг/сут), амлодипин (5 мг/сут), ацетилсалициловую кислоту (100 мг/сут).
Аллергологический анамнез: не отягощен.
Эпидемиологический анамнез: без особенностей.
Объективный статус при поступлении: Состояние средней тяжести. Температура 38,7 °С. На коже голеней единичные эритематозные папулы округлой формы. Видимые слизистые обычной окраски. Отеков нет. Лимфоузлы не увеличены. Форма грудной клетки нормостеническая. Дыхание через нос свободное. Частота дыхательных движений (ЧДД) 16/мин. Обе половины грудной клетки равномерно участвуют в акте дыхания. Пальпация грудной клетки безболезненна. Голосовое дрожание не изменено. Перкуторно над легочными полями ясный легочный звук. Аускультативно дыхание везикулярное, проводится во все отделы, хрипов нет. SpO2 на воздухе 96%. Область сердца не изменена. Левая граница относительной сердечной тупости на 1 см кнаружи от срединно-ключичной линии. Тоны сердца ясные. Шумы не выслушиваются. Ритм правильный, частота сердечных сокращений (ЧСС) 88 уд/мин, пульс 88/мин. АД (правая рука) 100/70 мм рт.ст., АД (левая рука) 100/70 мм рт.ст. Язык влажный, обложен белым налетом. Живот при пальпации мягкий, несколько болезненный. Печень не увеличена, при пальпации гладкая, эластичная. Селезенка не увеличена. Симптом «поколачивания» отрицательный с обеих сторон. Костно-суставная, мышечная система без патологии. Изменений неврологического статуса нет.
Данные лабораторных исследований от 25.02.2021:
- клинический анализ крови: эритроциты (RBC) – 4,06×1012/л; Hb – 126,4 г/л; тромбоциты (PLT) – 178×109/л; лейкоциты – 10,2×109/л; нейтрофилы палочкоядерные – 16%; нейтрофилы сегментоядерные – 65%; лимфоциты – 13,7%; нейтрофилы, абс. – 8,2×109/л; лимфоциты, абс. – 1,4×109/л; СОЭ – 58 мм/ч;
- биохимический анализ крови: глюкоза – 6,83 (3,6–6,3) ммоль/л; билирубин общий – 16,4 (5,0–21,0) мкмоль/л; аспартатаминотрансфераза (АСТ) – 20,9 (0,0–40,0) Ед/л; аланинаминотрансфераза (АЛТ) – 44,3 (0,0–40,0) Ед/л; креатинкиназа (КФК) – 108,4 (0,0–145,0) Ед/л; креатинин – 96,3 (53–97) мкмоль/л; общий белок – 77,4 (66,0–88,0) г/л; альбумин – 36,3 (33,0–53,0) г/л; лактатдегидрогеназа (ЛДГ) – 538 (0–480) Ед/л; СРБ – 296,6 (0,0–5,0) мг/л; мочевина 6,14 (2,5–8,3) ммоль/л; ферритин – 457 (15–150) мкг/л;
- коагулограмма: протромбин – 67,3 (70–130) %; фибриноген – 6,93 (1,8–4,0) г/л; активированное частичное тромбопластиновое время (АЧТВ) – 39,4 (24–35) с; международное нормализованное отношение (МНО) – 1,21 (0,9–1,1) у. ед.; Д-димер – 2,6 (0–0,5) мкг FEU/мл;
- прокальцитонин – 0,12 (0–0,5) мг/л;
- тропонин – отр.; Na-proBNP – 96 (<125) пг/ мл;
- РНК SARS-CoV-2 – не обнаружена;
- антитела к SARS-CoV-2 IgG – 10,6 (>1,1, положительно);
- исследование кала на токсины А и В C. difficile – отр.
Результаты (заключение) компьютерной томографии (КТ) органов грудной клетки от 25.02.2021: КТ-признаки двусторонней полисегментарной пневмонии с высокой степенью вероятности вирусной этиологии в стадии разрешения.
Данные ЭКГ и ЭхоКГ от 25.02.2021: без патологии.
Учитывая имеющиеся данные, а именно развитие заболевания через 4 нед после острого периода COVID-19 (диагноз перенесенной новой коронавирусной инфекции был подтвержден в остром периоде положительным результатом исследования мазка из рото- и носоглотки на РНК SARS- CoV-2, при текущей госпитализации – наличием антител IgG к SARS-CoV-2), фебрильную лихорадку в течение >3 дней, наличие симптомов поражения ЖКТ, кожи, гипотензию, повышение уровней маркеров воспаления (СРБ, ферритина, СОЭ), а также наличие признаков активности заболевания (лейкопения, нейтрофилия), пациентке был установлен диагноз «мультисистемный воспалительный синдром».
Проведенное лечение: метилпреднизолон по 500 мг/сут в течение 3 дней с последующим снижением дозы и переходом на прием внутрь; гепарин по 24 000–30 000 ЕД/сут внутривенно в течение 5 дней; далее эноксапарин, диокстаэдрический смектит, эзомепразол, эубиотик Bifidobacterium longum + Enterococcus faecium, инфузионная терапия. Лечение сопутствующей патологии – без изменений.
В динамике лихорадка была купирована в течение 3 сут, рвота и диарея – в течение суток. Динамика уровней лабораторных маркеров воспаления представлена на рисунке.
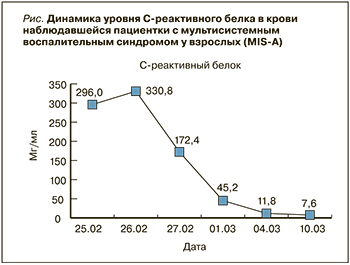
Пациентка была выписана 11.03.2021 в удовлетворительном состоянии.
ЗАКЛЮЧЕНИЕ
MIS-A относится к редким, но тяжелым и жизнеугрожающим осложнениям новой коронавирусной инфекции COVID-19. В представленном обзоре литературы мы постарались обобщить имеющиеся к настоящему времени данные о MIS-A (а также проиллюстрировать эти данные наглядным клиническим случаем), что позволит практикующему врачу своевременно заподозрить, диагностировать и лечить это отсроченное патологическое состояние, патогенетически связанное с дисрегуляцией иммунного ответа, эндотелиальной дисфункцией и гипериммунным воспалением, характерными для острого периода COVID-19. Для лучшего понимания патогенеза MIS-A необходимы дальнейшие исследования.



