ВВЕДЕНИЕ
Стресс (англ. stress – давление, напряжение) определяется как состояние, при котором гомеостаз организма находится под угрозой (или воспринимается как находящийся под угрозой) под влиянием сильных внешних или внутренних, в том числе психоэмоциональных воздействий [1–3]. Реакция организма на стресс, независимо от стрессора, универсальна и характеризуется стереотипными изменениями функции нервной и эндокринной систем. Термин «стресс» часто используется в различном контексте и может иметь разнообразные определения в зависимости от стрессора (травматический, окислительный, психологический и др.). Однако в научных исследованиях его определение довольно строгое: говорить о наличии стресса можно при активации симпатоадреналовой системы, являющейся неспецифическим признаком общего возбуждения вегетативной нервной системы, и при вовлеченности в процесс гипоталамо-гипофизарно-надпочечниковой (ГГН) оси [2, 4]. Основные конечные эффекторы реакции на стресс – высвобождение кортизола ГГН-системой и выделение катехоламинов (норадреналина и адреналина) периферической симпатоадреналовой системой, что и обусловливает его системное воздействие на организм [5, 6]. Медиаторы стресса нацелены на поведенческие, метаболические, сердечно-сосудистые, иммунные и желудочно-кишечные функции и их реакции [7–9].
Непродолжительное воздействие во времени или умеренное влияние факторов стресса активирует ряд физиологических функций: улучшается кровоснабжение мозга, повышается метаболизм, усиливаются зрение, слух, память, увеличивается сердечный выброс. Все это позволяет организму адаптироваться к стрессу без ущерба для здоровья. Данная реакция адаптивная и носит название «эустресс» [3]. Что же касается интенсивного и длительного стресса, который превосходит адаптивные возможности организма, то он называется «дистресс» или «хронический стресс». Этот вариант вызывает патофизиологические реакции и может способствовать развитию хронических заболеваний, среди которых болезни сердечно-сосудистой системы занимают центральное место.
Гомеостаз во время стресса поддерживается и восстанавливается сложным взаимодействием поведенческих и физиологических адаптивных реакций различных органов и систем. В последние годы понятие гомеостаза было дополнено в работах П. Стерлинга и Дж. Эйера и расширено исследованиями Бр. Макьюэна [10]. Термин «гомеостаз», который подразумевает постоянство внутренней среды, сохраняющееся через оптимальное соотношение ее параметров, дополнен понятием «аллостаз». Аллостаз – это активный процесс адаптации к стрессорам с помощью как медиаторов ГГН оси, так и медиаторов вегетативной, метаболической и иммунной систем, действующих взаимосвязано, но нелинейно, и направленных на поддержание гомеостаза. Понятие аллостатической нагрузки относится к кумулятивному эффекту множественных стрессоров, а также к нарушению регуляции нелинейных связей (например, слишком много или слишком мало кортизола, адреналина или воспаления в ответ на стрессор), что может привести к нейробиологическим нарушениям и преждевременному старению [1, 10, 11]. Очень интересны и актуальны исследования по распространению стресса среди населения, возможности его передачи от человека к человеку [1, 12].
В последние годы проводится множество исследований, пытающихся ответить на вопросы: откуда может возникнуть ощущение постоянного «напряжения» и почему одни люди справляются лучше со стрессовой ситуацией, чем другие? Как это влияет на мозг и сердечно-сосудистую систему? Каковы стратегии борьбы со стрессом? С целью отбора и последующего анализа нами были рассмотрены соответствующие публикации, размещенные в базах данных PubMed, MEDLINE, Cochrane Library databases с 2011 по 2021 г., а также ряд более ранних, основополагающих работ по интересующей нас теме. В итоге для обзора были выбраны исследования, отвечающие классам и уровням доказательств IA и IB, IIA (рандомизированные клинические исследования, когортные исследования, метаанализы, систематические обзоры). Цель обзора – показать современные направления исследований стресса и применение их результатов в клинической практике.
ФИЗИОЛОГИЯ И НЕЙРОБИОЛОГИЯ СТРЕССА, ЕГО КЛИНИЧЕСКОЕ ЗНАЧЕНИЕ
Многочисленные факторы, вызывающие стресс, условно можно разделить на две большие группы [3].
1. Физические стрессоры, непосредственно влияющие на тело, такие как боль, инфекция, холод или жара, гипоксия, шум, голод/отсутствие пищи, тяжелая физическая работа, потеря крови и т.п. Они непосредственно активируют различные группы соматических сенсорных рецепторов: баро-, волюмо-, хеморецепторы, рецепторы боли и др. Далее сигналы передаются по сенсорным путям в различные отделы центральной нервной системы (ЦНС).
2. Эмоциональные стрессоры – неожиданная ситуация или обстановка, давление обстоятельств, ужас, страх, беда, напряжение отношений в семье или на работе и прочие психосоциальные факторы. Сигналы от таких стрессоров первично оцениваются в лимбической системе (гиппокамп, миндалевидные тела) и обрабатываются в префронтальной коре, в дальнейшем активируя ГГН-ось. В данной ситуации важны анализ и память предыдущего опыта стрессовых ситуаций, влияющие на выраженность адаптивной реакции организма. Важно отметить, что само ожидание возможных проблем, особенно если они носят социальный или психологический характер, может активировать стрессовую реакцию, изменения в поведении [13].
Восприятие мозгом событий после обработки сенсорных, когнитивных и эмоциональных сигналов как факторов стресса запускает различные физиологические и поведенческие реакции. При этом вторично, реальна ли опасность, или же имеет место только страх перед возможной опасностью; на различные стрессоры организм отвечает похожими реакциями, что убедительно подтверждено в многочисленных исследованиях [1,3, 7,11]. Реакции организма на адаптацию к стрессу включают:
- повышение внимания, быстроты обработки информации, улучшение памяти;
- мобилизацию энергетических резервов, поскольку при стрессе важно обеспечить энергией мозг и мышцы;
- повышение частоты дыхания и активацию сердечно-сосудистой системы;
- модуляцию иммунных функций;
- блокаду вегетативных функций, таких как потребность в сексе и голоде;
- задержку воды при потере крови.
Центральные эффекторные системы быстрого ответа на стресс находятся в гипоталамусе и стволе головного мозга. К ним принадлежат секретирующие кортикотропин-релизинг (КРГ) и антидиуретический гормоны нейроны мелкоклеточной части Nucleus paraventricularis гипоталамуса и норадренергические нейроны центральной симпатической системы (Locus coeruleus) ствола мозга [3, 14]. В результате через активацию эндокринной ГГН-оси и симпатоадреналовой системы вырабатываются основные периферические эффекторы стресса – кортизол, адреналин и норадреналин, которые оказывают основное системное патофизиологическое и клиническое воздействие, а при длительном (хроническом) стрессе формируют негативный эффект для органов и систем. Катехоламины посредством активации β2-адренорецепторов в иммунных клетках и клетках гладких мышц стимулируют секрецию интерлейкина 6 (ИЛ-6), играющего важную роль в развитии аутоиммунного воспаления.
Хронический стресс характеризуется длительной гиперактивностью ГГН-оси [5, 8]. Фоновая и стресс-индуцированная секреция кортизола в этом случае повышена, что влияет на ослабление чувствительности обратной связи между уровнем этого гормона в крови и секрецией КРГ. Постоянное повышение концентрации в крови кортизола уже не блокирует выработку гипоталамусом КРГ по механизму обратной связи. Повышенный уровень глюкокортикоидов при длительном воздействии оказывает негативное влияние не только на структуры гипоталамуса, но и другие отделы мозга, содержащие глюкокортикоидные рецепторы; среди них особое, центральное место занимает гиппокамп, участвующий в нейрогенезе и формировании памяти. Кроме того, хорошо проникающий через гематоэнцефалический барьер кортизол повышает чувствительность к стрессу рецепторов центральных ядер миндалевидного тела головного мозга, что клинически связано с усилением чувства тревожности и страха у пациентов [15, 16].
Для хронического стресса характерны следующие дезадаптивные реакции:
- снижение памяти и внимания, нарушение сна;
- повышение артериального давления (АД), частоты сердечных сокращений (ЧСС);
- увеличение веса, окружности талии;
- гипофункция щитовидной железы;
- нарушение обмена глюкозы;
- иммуносупрессия;
- дисфория, депрессия, сексуальные нарушения.
В работах нейробиолога Sapolsky R.M. показано, что скорость и сила ответа симпатической нервной системы и синтез кортизола могут меняться в зависимости от стрессора и предшествующего опыта преодоления стресса, создавая особый гормональный «рисунок индивидуального стресса» и формируя его индивидуальную переносимость [13]. Ответ на стресс ассоциирован с генетическими и эпигенетическими факторами. Влияние предшествующего опыта преодоления стресса связывают с эпигенетическими механизмами регуляции ответа – метилированием цитозина в цитозин-гуанин-динуклеотиде (ЦГД) ДНК, которое ведет к уменьшению активности промоторной части гена, влияющей на транскрипцию. В настоящее время активно изучаются факторы окружающей среды, образа жизни, гормоны, способные оказывать воздействие на формирование эпигенетических механизмов. Все это важно не только для понимания механизмов стресса, но и разработки лекарственных препаратов, однако многие вопросы продолжают оставаться открытыми [11, 17]. В ряде исследований подчеркивается, что, как правило, недостаточная адаптация чаще всего отмечается на похожие типы стрессоров. В то же время с возрастанием числа стрессовых событий, предшествующего положительного опыта адаптации к стрессу реакция на него становится слабее [18].
ВЛИЯНИЕ СТРЕССА НА МОЗГ
Мозг служит мишенью различных факторов стресса. Глюкокортикоиды совместно с возбуждающими аминокислотными нейротрансмиттерами меняют архитектуру нейронов, вызывая сокращение или расширение дендритов, снижение или увеличение плотности синапсов в зависимости от области мозга, наряду с ингибированием нейрогенеза зубчатой извилины гиппокампа [10]. Различные межклеточные медиаторы и процессы участвуют в изменении структуры и функции мозга во время стресса, а также в его восстановлении после прекращения воздействия факторов стресса (табл.).
Стрессоры меняют экспрессию генов в нейронах с помощью различных механизмов, включая прямое воздействие глюкокортикоидов на транскрипцию генов и активацию эпигенетических механизмов, в которых основную роль играют модификации гистонов и метилирование / гидроксиметилирование остатков цитозин-гуанина в ДНК [19]. Глюкокортикоиды – не единственные медиаторы этих эффектов, также большое значение в их развитии имеют возбуждающие аминокислоты мозга (глутамат) и другие клеточные медиаторы (см. табл.).
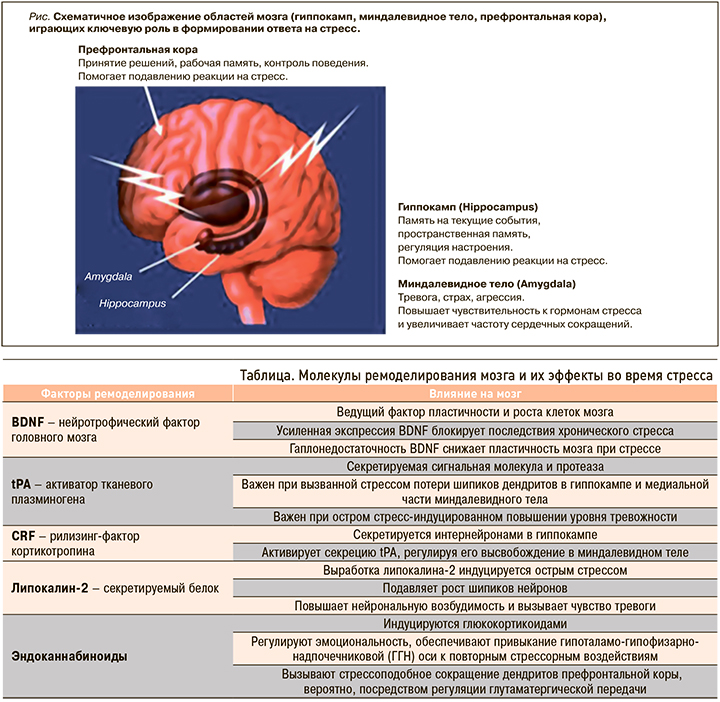
Стресс оказывает широкое влияние на весь мозг. Три области мозга – гиппокамп, миндалевидные тела и префронтальная кора, играющие важную роль в поведении и когнитивных функциях, а также в регуляции вегетативной нервной системы и ГГН-оси, занимают особое место и при формировании реакций на стресс (рис.).
1. Гиппокамп. Глюкокортикоидные и минералокортикоидные рецепторы обнаружены в гиппокампальной формации, что свидетельствует о влиянии надпочечниковых стероидов на мозг не только через связи с гипоталамусом, но и, как мы теперь знаем, непосредственно на гиппокамп – структуру, участвующую в формировании пространственной и эпизодической памяти, регуляции настроения, нейрогенезе [3, 10]. Именно в работах, изучающих гиппокамп, впервые было продемонстрировано негативное воздействие стресса на мозг. В гиппокампе стресс и эффекторы стресса глюкокортикоиды вызывают сморщивание дендритов и потерю ими шипиков, очень важных при контактах между нейронами, обмене информации, обучении моторным функциям. В основе снижения памяти при стрессе лежит разобщение связей между клетками неокортекса больших полушарий мозга и нейронами энториальной коры гиппокампа под воздействием медиаторов стресса. Разрыв нейрональных связей приводит к нарушению обмена и хранения информации, снижению скорости ее обработки, что влечет за собой негативные последствия для памяти [20, 21]. Полученные в более ранних исследованиях подтверждения нейрогенеза в зубчатой извилине гиппокампа стимулировали интерес к изучению данной области. Показано, что длительный хронический стресс может вызывать атрофию гиппокампа, нарушая процесс нейрогенеза и ограничивая возможности нейропластичности мозга. В том же гиппокампе впервые была показана в исследованиях и роль возбуждающих аминокислот в реализации стрессовых эффектов, обусловливающих эксайтотоксическое влияние на мозг. Возбуждающие аминокислоты, особенно глутамат, вносят ключевой вклад в структурные, а также функциональные изменения в головном мозге. В первоначальных исследованиях стресса было установлено, что при его хроническом воздействии потеря апикальных дендритов нейронов зоны нейрогенеза гиппокампа связана с повышением внеклеточного уровня глутамата [22]. Нужно отметить, что блокирование рецепторов NMDA глутамата и вмешательство в возбуждающую стимуляцию ионных каналов кальция, блокирует вызванное стрессом ремоделирование дендритов в гиппокампе. Глюкокортикоиды могут напрямую стимулировать высвобождение возбуждающих аминокислот через ассоциированные с мембраной нейронов рецепторы или опосредованно регулировать высвобождение глутамата через индукцию локального синтеза эндоканнабиноидов мозгом [10, 23] (см. табл.).
2. Миндалевидное тело. Воздействие острого и хронического стресса на эту область мозга отличается от действия на гиппокамп. Обнаружено, что острые стрессоры приводят к увеличению плотности шипиков на нейронах базолатеральных ядер миндалины, а хронический стресс – к утолщению базолатеральных дендритов миндалевидного тела. Эти изменения связывают с повышенной тревожностью у пациентов и поведением, подобным посттравматическому стрессу [24, 25]. Миндалевидное тело отвечает за такие наши эмоции, как чувство испуга, страха, агрессии, принятие эмоциональных решений.
3. Префронтальная кора. В этой области мозга, отвечающей за стратегическое планирование, контроль действий и принятие решений, хронический стресс вызывает сокращение количества дендритов медиальных нейронов, что связывают с развитием когнитивной ригидности у человека. В то же время нейроны орбитофронтальной коры, участка префронтальной коры лобных долей мозга, увеличивают количество дендритов, и это может быть ассоциировано с повышенной бдительностью во время стресса. В исследованиях показано, что устойчивость реакций префронтальной области к стрессу снижалась с возрастом, а также при нарушении циркадных ритмов сна и бодрствования [26].
Циркадные (или суточные) ритмы относятся к весомым факторам, определяющим реакцию стресса. Ритмическая функция КРГ, по-видимому, необходима для нормальной инициации и прекращения действия адренокортикотропного гормона и других медиаторов стресса. Эпидемиологические исследования показали, что нарушение сна и циркадных ритмов у посменных рабочих или групп населения, хронически подвергающихся таким нарушениям, сопряжены с повышенным риском развития психических, сердечно-сосудистых или других патофизиологических синдромов [27]. Интересно, что глюкокортикоиды способны регулировать экспрессию генов «циркадных часов» в нескольких областях мозга [10, 28, 29].
Ассоциация между повышенной частотой цереброваскулярной патологии и хроническим стрессом убедительно показана в клинических и экспериментальных исследованиях. Так, в метаанализе, включившем 399 791 участника, было продемонстрировано, что стресс и депрессия связаны со значительно повышенным риском инсульта [3]. Постоянное негативное воздействие психологических факторов, приводящих к стрессу, повышает угрозу развития инсульта на 45%. При этом инсульт, развивающийся на фоне стресса, протекает особенно тяжело и чаще заканчивается летальным исходом [31].
Воздействие стресса на мозг поддается коррекции [32]. Для таких заболеваний, как депрессия и тревожные расстройства, включая посттравматическое стрессовое расстройство (ПТСР), «первой линией» лечения служит когнитивно-поведенческая терапия, которую необходимо дополнять медикаментами и стратегиями, которые открывают «окна пластичности мозга», повышая эффективность поведенческих вмешательств [33].
ВЛИЯНИЕ СТРЕССА НА СЕРДЕЧНО-СОСУДИСТУЮ СИСТЕМУ
Стресс провоцирует выброс катехоламинов, возбуждающих α- и β-рецепторы в сердечно-сосудистой системе. Эволюционно физиологические реакции, сопутствующие стрессу, подготавливают организм для предстоящей деятельности, и в первую очередь для физической активности. Однако в современных условиях двигательные реакции в ответ на стрессовые факторы чаще всего подавляются, что приводит к разрыву эволюционно закрепленного комплекса соматических и вегетативных реакций [3, 12].
Стрессовая ситуация, не сопровождающаяся включением скелетных мышц, вызывает более выраженное повышение АД из-за отсутствия рабочей гиперемии, более продолжительное сохранение высоких концентраций адреналина, липидов и глюкозы в крови. При продолжительном воздействии стрессового фактора несоответствие между избыточным ответом и малыми потребностями организма может стать причиной развития сердечно-сосудистых заболеваний (ССЗ), в том числе стойкого повышения АД [6, 13].
В развитии артериальной гипертензии (АГ) необходимо различать факторы, непосредственно приводящие к подъему АД, и факторы, принимающие участие в долгосрочной его регуляции на повышенном уровне. Подъем АД, связанный с действием стрессового фактора, в основном обусловлен активацией симпатической нервной системы, в то время как длительное повышение АД на фоне хронического стресса и переход его на новый уровень регуляции в большей степени обусловлено морфологическими изменениями сосудистой стенки с повышением сопротивления и изменением характеристик потока крови, а также нарушением функции эндотелия, изменением соотношения сосудорасширяющих и сосудосуживающих веществ [8]. Один из наиболее значимых факторов, принимающих участие в регуляции сосудистого тонуса, – оксид азота (NO). Степень его секреции эндотелием тесно связана с уровнем АД и характеристикой потока крови, степенью напряжения сдвига на сосудистую стенку. Эндотелий сосудов принимает участие в регуляции сосудистого тонуса, агрегации тромбоцитов и других процессов, влияющих на развитие атеросклероза. Развитие эндотелиальной дисфункции в первую очередь приводит к ухудшению расширения сосудов. Состояние эндотелия тесно связано с гемодинамическим ответом на стрессовую реакцию, и у испытуемых с нарушенной эндотелиальной функцией отмечается большее повышение периферического сосудистого сопротивления в ответ на психоэмоциональную нагрузку [8, 34]. Это объясняется в том числе вовлеченностью NO в механизмы эмоционального стресса. Блокада NO-синтетазы повышает чувствительность субъектов к стрессовым факторам, а дефицит NO обусловливает более выраженное сужение кровеносных сосудов под влиянием медиаторов, вследствие чего формируется устойчивая АГ, нарушаются функции сердца, почек и других органов [34].
Немалое значение в развитии АГ на фоне стресса имеют генетические факторы и возможность человека адаптироваться к продолжительной стрессовой ситуации. Современные исследования свидетельствуют, что психоэмоциональное состояние пациента недостаточно оценивается в клинической практике как фактор, влияющий на эффективность лечения и прогноз различных заболеваний. При этом негативное психологическое состояние приводит к специфическим биологическим изменениям в организме: в частности, хронический стресс связан с гиперкоагуляцией, дислипидемией, усилением воспалительных процессов, нарушением иммунных реакций и контроля глюкозы [35, 36].
Исследования последних лет четко выявили связь психосоциального стресса и состояния сердечно-сосудистой системы. По данным Американской ассоциации кардиологов, такие негативные факторы как хронический стресс, тревога, депрессия, гнев, пессимизм и неудовлетворенность своей жизнью связаны с возрастанием распространенности ССЗ.
К настоящему времени опубликованы два крупных метаанализа связи ПТСР и ССЗ, последний из которых был проведен в 2018 г. и включал 9 исследований с участием 151 144 участников. Анализ результатов показал, что ПТСР был связан с повышением риска развития ишемической болезни сердца (ИБС) на 61%. Эта ассоциация оставалась значимой и после дополнительной корректировки данных на сопутствующую депрессию [37].
В более раннем метаанализе (6 исследований, 118 696 участников) основное внимание было уделено восприятию пациентами стресса независимо от вызвавшей его причины. В нем было установлено, что высокое восприятие стресса ассоциировано с увеличением риска развития ИБС на 27% [38]. В другом метаанализе было продемонстрировано, что связанный с работой высокий уровень воспринимаемого стресса приводит к повышению вероятности развития сердечно-сосудистых заболеваний на 40% [39].
В нескольких метаанализах изучалась связь тревоги и ССЗ. Крупнейшее исследование включало 2 017 276 участников из 46 когорт. Тревожность была связана как с повышенным риском смертности от ССЗ, так и с развитием специфических типов ССЗ, включая ИБС, инсульт и сердечную недостаточность [40]. В одном исследовании была показана ассоциация стресса со значительным повышением риска возникновения спазма коронарной артерии, в другом у пациентов с острым коронарным синдромом (ОКС) была выявлена зависимость «доза–реакция» между тяжестью депрессии и снижением приверженности к терапии (15% у пациентов без депрессии, 29% – с легкой депрессией, 37% – с умеренной и тяжелой депрессией) [41].
Существует зависимость также между депрессией и риском смертности от различных ССЗ. В ряде метаанализов обнаружена связь между депрессией и повышенным риском инфаркта миокарда и ИБС. Эти ассоциации оставались значимыми после коррекции потенциальных факторов риска, влияющих на ситуацию, включая социально-демографические и поведенческие факторы [42]. Хотя эти исследования были сосредоточены на случаях с первичными инцидентами ССЗ, убедительные данные также свидетельствуют о том, что депрессия увеличивает угрозу повторных событий и смертности у кардиологических пациентов [43].
Недавние продолжительные исследования убедительно доказывают, что такие факторы, как депрессия и ПТСР, усиливают воспалительные реакции как у здоровых людей, так и пациентов, имеющих ССЗ, способствуя прогрессированию атеросклероза и повышая риск артериального тромбоза [44]. Известно, что такие факторы риска ССЗ, как курение, низкий уровень физической активности, плохое качество питания и избыточный вес увеличивают чувствительность глюкокортикоидных рецепторов к кортизолу и снижают устойчивость к стрессу [45].
В заключение этого раздела необходимо отметить, что адаптация сердечно-сосудистой системы к стрессу зависит от характера стрессорных факторов. Нормализация ее показателей после перенеенного психоэмоционального стресса обычно продолжается более длительное время, чем после аналогичного по величине воздействия стресса физического. Таким образом, в настоящее время получено достаточное количество доказательств, подтверждающих достоверность причинно-следственной связи между психологическим состоянием пациента и риском развития и прогрессирования у него ССЗ посредством биологических, поведенческих и психосоциальных механизмов.
ВОЗМОЖНОСТИ ДИАГНОСТИКИ СТРЕССА
На основании накопленных данных показана возможность лабораторной диагностики стресса [7, 46]. Сложность воздействия стресса на здоровье требует объективизации не только физиологических, но и психологических его индикаторов. В современных стандартах ведения пациентов с ССЗ и цереброваскулярными заболеваниями практическим врачам рекомендуется обращать внимание не только на лечение основного заболевания, но и на психическое здоровье пациента [47].
Кортизол служит главным биомаркером стресса. Его секреция изменчива в течение суток, что обусловлено физиологическим суточным ритмом этого гормона (колебание уровней от утренних часов к вечерним), а также определенными воздействиями на организм в течение дня различных факторов (никотина, алкоголя, приема пищи, интенсивных физических упражнений, острых психосоциальных ситуаций). Уровень кортизола обычно наиболее высок при пробуждении, быстро падает в последующие несколько часов, затем медленно снижается, достигая наименьшей концентрации перед сном [48]. Суточный ритм кортизола при исследовании разделяют на несколько ключевых компонентов, которые позволяют повысить точность диагностики стресса. Наиболее часто исследуются средний уровень кортизола в течение дня (daily average cortisol – DAC), уровень всплеска после пробуждения (called the cortisol awakening response – CAR), суточная кривая кортизола (the diurnal cortisol slope – DCS), отражающая степень изменения его концентрации от утра к вечеру в период бодрствования [49].
Измерения кортизола в плазме, слюне или моче доказали свою достоверность при диагностике острого стресса, но практически не информативны при стрессе хроническом. Накапливающийся объем исследований последних 15 лет, посвященных суточным кривым кортизола (DCS), показывает, что при диагностике хронического эмоционального и психосоциального стресса именно этот метод наиболее чувствителен и специфичен. Интересно, что более плоская кривая DCS в течение суток отражает наиболее неблагоприятные исходы заболевания [49].
В систематическом обзоре и метаанализе 2017 г., который был основан на 80 исследованиях, включавших 36 823 участника, выявлена достоверная связь между наиболее плоскими DCS и более тяжелым состоянием эмоционального и физического здоровья во всех исследованиях. Самая сильная связь была обнаружена между кривой DCS и выраженностью иммунных и воспалительных исходов.
Таким образом, суточные кривые кортизола (DCS) были предложены в качестве маркера влияния хронического психосоциального стресса на психическое и физическое здоровье. При этом более плоские DCS содержат элементы как гипо- так и гиперкортизолизма (включая низкий утренний и/или высокий вечерний уровни кортизола). Высокий базальный уровень кортизола, особенно в вечерние часы, был связан с депрессией, в то время как более низкий базальный уровень этого гормона – с расстройствами пищевого поведения [49].
Недавней методологической разработкой является анализ концентрации кортизола в волосах (Hair Cortisol Concentrations – HCC), который становится все более важным методом оценки в психонейроэндокринологических исследованиях. НСС обеспечивает легко получаемый показатель уровня кортизола за длительный период времени (несколько месяцев роста волос). В настоящее время анализ НСС подтвердил свою достоверность, высокую надежность в повторных испытаниях и стабильность при воспроизведении результатов [50].
Stalder T. et al. [50] проанализировали 124 выборки, включавшие 10 289 участников, и констатировали, что в группах с хроническим стрессом отмечалось увеличение НСС на 22%. Также результаты метаанализа продемонстрировали положительную связь НСС с антропометрическими показателями, связанными со стрессом (индекс массы тела, соотношение талии и бедер) и гемодинамическими параметрами (систолическое АД).
Исследования последних лет по изучению альфа-амилазы слюны (Saliva Alpha Amylase – sAA) как диагностического маркера стресса (уровень активности симпатической нервной системы) не подтвердили свою надежность [51, 52]. В настоящее время показано, что активность sAA связана с активацией как симпатической, так и парасиматической нервной системы, что ограничивает применение этого маркера для диагностики стресса [7, 53].
Поиск новых биомаркеров стресса – постоянно развивающаяся область исследований в психонейроэндокринологии. Наряду с кортизолом изучаются также дополнительные метаболиты оценки уровня хронического стресса, такие как BDNF, ИЛ-6, ИЛ-8, ИЛ-1-β, С-реактивный белок, фактор некроза опухоли-альфа. Недостаточно информативны при диагностике стресса определение уровней в крови антиоксидантов и естественных клеток-киллеров (NK) [7, 46], что требуют дальнейших исследований в этом направлении.
Таким образом, с целью комплексной диагностики состояния пациента имеется достаточный лабораторный выбор биомаркеров стресса, которые могут использоваться врачами для оценки влияния хронического стресса на физическое и психическое здоровье.
КОРРЕКЦИЯ СТРЕССА: ДОКАЗАТЕЛЬНАЯ БАЗА
Основные терапевтические стратегии коррекции стресса направлены на его клинические симптомы. К наиболее частым последствиям стресса, помимо обострения имеющейся соматической патологии, относятся различные негативные стресс-ассоциированные состояния: тревога, повышенная раздражительность, плохой сон, агрессия, гнев, немотивированное ощущение страха, депрессия. Отдельное место занимают диагностика и лечение ПТСР [54–56].
На прогноз и выбор тактики лечения основного заболевания оказывают влияние психические и поведенческие формы стресс-ассоциированных расстройств, которые важно выявлять врачу первичного звена. В терапевтическом процессе рекомендуется применяться различные количественные опросники, оценивающие процесс лечения и изменение психического состояния пациента. Наиболее часто в клинической практике используются следующие скрининговые шкалы и опросники [11]:
- «Госпитальная шкала тревоги и депрессии», легко применима в условиях общемедицинской практики. Она позволяет провести скрининг субклинических и легких проявлений стресс-связанных расстройств (тревоги и депрессии);
- «Тест самооценки стрессоустойчивости С. Коухена и Г. Виллиансона», при помощи которого пациент может сам оценить свое состояние и при необходимости обратиться к доктору;
- «Шкала воспринимаемого стресса-10» (The Perceived Stress Scale-10, PSS-10), позволяющая определить интенсивность полученного стресса за последний месяц;
- Стресс-тест по Триер (Trier Inventory for Chronic Stress, TICS) – высоконадежный и достоверный опросник в оценке хронического стресса.
Наибольшую доказательную базу (уровень и сила доказательности IA) в лечении стресса и стресс-ассоциированных расстройств имеют различные виды психотерапии, фармакотерапии, психофармакотерапия (сочетание лекарственной терапии и психотерапии). Наилучший эффект показало сочетание когнитивно-поведенческой психотерапии и антидепрессантов [54, 57, 58].
Антидепрессанты из группы селективных ингибиторов обратного захвата серотонина (СИОЗС) рассматриваются как «первая линия» медикаментозной коррекции большинства расстройств, связанных со стрессом. Исследования показали, что препараты этого класса безопасны и эффективны в лечении серьезных депрессивных расстройств у пациентов с ССЗ и в профилактике нарастания степени тяжести депрессии [59–61]. В более поздних плацебо-контролируемых исследованиях продолжилось изучение влияния антидепрессантов непосредственно на сердечно-сосудистые исходы. Так, в исследовании ESDEPACS выполнялось длительное наблюдение (8 лет) за пациентами с депрессией после ОКС. Было установлено, что в группе пациентов, принимавших СИОЗС эсциталопрам, показатель рецидива ССЗ был более низким по сравнению с группой, получавшей плацебо (40,9 против 53,6%; относительный риск 0,69; 95% доверительный интервал: 0,49–0,96) [62]. Результаты контролируемых исследований 2013 г. продемонстрировали, что прием СИОЗС дает дополнительный эффект улучшения вариабельности сердечного ритма и ишемии миокарда на фоне основной терапии у пациентов с ССЗ [63]. Негативного влияния СИОЗС на исходы ССЗ в проведенных исследованиях выявлено не было [47]. В связи с этим можно заключить, что антидепрессанты группы СИОЗ хорошо переносятся пациентами с острыми и хроническими сердечно-сосудистыми заболеваниями, способствуя при этом сопутствующему улучшению результатов психического здоровья.
Препараты группы бензодиазепинового ряда назначаются преимущественно при остром стрессе как скоропомощные препараты для кратковременного купирования тревоги, паники, нарушений сна и не рекомендуются для длительного использования во время хронического стресса [54, 64].
В отличие от методов лечения, которые непосредственно направлены на коррекцию психического состояния пациента (психотерапия, антидепрессанты), другие вмешательства нацелены на общий уровень снижения стресса, активное содействие позитивному психоэмоциональному состоянию (например, ощущение счастья, осознанность, благодарность) и относятся к группе адъювантных методов лечения при стрессе [65, 66]. К таким практикам относятся прогрессивная мышечная релаксация, аутогенная тренировка, различные виды йоги, дыхательные техники с урежением частоты дыхания, аэробная физическая нагрузка. Адъювантые методы лечения в настоящее время имеют доказательную базу на уровне консенсуса экспертов [67, 68]. Вместе с тем исследования убедительно показывают увеличение синтеза BDNF, имеющего значительное влияние на улучшение пластичности мозга и повышение устойчивости к стрессу, при аэробных физических нагрузках [20, 23]. Различные техники йоги, направленные на медитацию и снятие мышечного напряжения, достоверно снижают уровень накопления кортизола в волосах [69, 70].
Для лучшего понимания влияния положительных и отрицательных факторов психического здоровья на механизмы развития ССЗ и их исходы необходимо дальнейшее продолжение исследований, несмотря на сложность методологической задачи. Возможность сочетания лечения сердечно-сосудистой патологии и психологической коррекции состояния пациента остается актуальным вопросом современной клинической практики и широко обсуждается медицинской общественностью.



